Abstract
Purpose
Fractional exhaled nitric oxide (FeNO) has emerged as an important biomarker in asthma. Increasing evidence points to atopy as a confounding factor in the interpretation of elevated FeNO. We conducted a longitudinal study to understand the clinical significance of FeNO as an inflammatory biomarker.
Methods
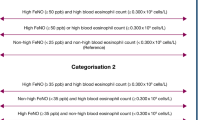
We identified 19 children aged 13–15 years at baseline with a significant elevation in FeNO ≥ 80 parts per billion (ppb) and randomly selected a group of children of similar age with a moderate elevation (40–79 ppb) and normal-to-low FeNO (<40 ppb). Between November 2010 and July 2011, three additional study visits were conducted.
Results
Ninety-three children participated in the study. There were 16, 24, and 53 participants in the high, mid, and low FeNO groups. During 1.5 years of follow-up, mean FeNO levels were 82.6 ppb (standard deviation [SD] = 65.9) for atopic asthmatics, 50.6 ppb (SD = 42.6) for nonasthmatic atopics, 17.0 ppb (SD = 10.8) for nonatopic asthmatics, and 17.8 ppb (SD = 13.9) for nonatopic nonasthmatics (p < 0.001). FeNO levels remained stable: 63 % of the high FeNO group had a FeNO ≥ 80 across all 4 measurements and 87 % of the normal-to-low FeNO group had a FeNO of <40 across all 4 measurements. The high FeNO group also was found to have an elevation in IL-5 (p = 0.04), IL-6 (p = 0.003), IL-10 (p = 0.002), and total serum IgE (p < 0.001), after adjustment by age, sex, height, body mass index, and atopy and asthma status.
Conclusions
An elevation of FeNO appears to indicate an atopic phenotype regardless of an asthma diagnosis, clinical symptoms, or corticosteroid use. An elevation of FeNO also is associated with a systemic elevation in inflammatory cytokines.




Similar content being viewed by others
References
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. http://www.ginasthma.org/local/uploads/files/GINA_Report_2012Feb13.pdf
Alving K, Weitzberg E, Lundberg J (1993) Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 6:1368–1370
Kharitonov S, Yates D, Barnes P (1996) Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med 153:454–457
Kharitonov S, Chung K, Evans D, O’Connor BJ, Barnes PJ (1996) Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med 153:1773–1780
Scott M, Raza A, Karmaus W (2010) Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 65:258–262
Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A (2006) Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med 174:1286–1291
Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, Shields MD (2002) Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 57:383–387
Sippel JM, Holden WE, Tilles SA et al (2000) Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol 106:645–650
Gelb AF, Flynn TC, Shinar CM et al (2006) Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest 129:1492–1499
Buchvald F, Eiberg H, Bisgaard H (2003) Heterogeneity of FeNO response to inhaled steroid in asthmatic children. Clin Exp Allergy 33:1735–1740
de Bot CM, Moed H, Bindels PJ, van Wijk RG, Berger MY, de Groot H, de Jongste JC, van der Wouden JC (2013) Exhaled nitric oxide measures allergy not symptoms in children with allergic rhinitis in primary care: a prospective cross-sectional and longitudinal cohort study. Prim Care Respir J 22:44–50
Dupont LJ, Demedts MG, Verleden GM (2003) Prospective evaluation of the validity of exhaled nitric oxide for diagnosis of asthma. Chest 123:751–756
Crane J, Lampshire P, Wickens K, Epton M (2012) Asthma, atopy and exhaled nitric oxide in a cohort of 6-yr-old New Zealand children. Pediatr Allergy Immunol 23:59–64
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR (2011) An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med 184:602–615
Robinson CL, Baumann LM, Romero K, Combe JM, Gomez A, Gilman RH, Cabrera L, Gonzalvez G, Hansel NN, Wise RA, Barnes KC, Breysse PN, Checkley W (2011) Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 66:1051–1057
Poon AH, Eidelman DH, Martin JG, Laprise C, Hamid Q (2012) Pathogenesis of severe asthma. Clin Exp Allergy 42:625–637
Lykouras D, Sampsonas F, Karparianos A, Karkoulias K, Spiropoulos K (2008) Role and pharmacogenomics of TNF-alpha in asthma. Mini Rev Med Chem 8:934–942
Desai D, Brightling C (2009) Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol 158:10–19
Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW (2001) Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol 125:177–183
John M, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, Barnes PJ, Chung KF (1998) Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am J Respir Crit Care Med 157:256–262
Robinson CL, Baumann LM, Gilman RH, Romero K, Combe JM, Cabrera L, Hansel NN, Barnes K, Gonzalvez G, Wise RA, Breysse PN, Checkley W (2012) The Peru Urban versus Rural Asthma (PURA) Study: methods and baseline quality control data from a cross-sectional investigation into the prevalence, severity, genetics, immunology and environmental factors affecting asthma in adolescence in Peru. BMJ Open 2(1):e000421
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) ATS/ERS Task Force: standardization of spirometry. Eur Respir J 26:319–338
Hankinson JL, Odencrantz JR, Fedan KB (1999) Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159:179–187
Linhares D, Jacinto T, Pereira AM (2011) Effects of atopy and rhinitis on exhaled nitric oxide values—a systematic review. Clin Transl Allergy 1:8
Dweik RA, Sorkness RL, Wenzel S (2010) Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med 181:1033–1041
Choi BS, Kim KW, Lee YI, Park HB, Kim YH, Sohn MH, Kim KE (2011) Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci 26:1265–1269
Sutherland TJ, Taylor DR, Sears MR (2007) Association between exhaled nitric oxide and systemic inflammatory markers. Ann Allergy Asthma Immunol 99:334–339
Ingram JL, Kraft M (2012) IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol 130:829–842
Wang XQ, Hu GH, Kang HY, Shen Y, Hong SL (2013) Significance of the IL-6 pathway in nasal polyposis in Chinese patients. Asian Pac J Allergy Immunol 31:11–19
Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ (2007) Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 8:1095–1104
Acknowledgments
This study was supported in part by the Johns Hopkins Center for Global Health. Mary Elmasri and Karina Romero were Fogarty International Center Research Fellows during the conduct of this work (R25TW009340). Colin Robinson was a Fogarty International Clinical Research Scholar during the time of this work and was further supported by Tufts University School of Medicine. Lauren Baumann was supported by a pre-doctoral NIH T35 Training Grant (T35AI065385). Nadia Hansel and William Checkley were supported by a R01 grant from the National Institutes of Environmental Health Sciences (R01ES018845). William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health and by a contract (HHSN268200900033C) with the National Heart, Lung and Blood Institute, National Institutes of Health. Study sponsors played no role in the study design, data collection, data analysis, data interpretation or the decision to submit the article for publication.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Other PURA Study Investigators Include
Juan Combe (A.B. PRISMA, Lima, Peru), Alfonso Gomez (A.B. PRISMA, Lima, Peru), Guillermo Gonzalvez (PAHO Lima, Peru), Lilia Cabrera (A.B. PRISMA, Lima, Peru), Kathleen Barnes (Johns Hopkins University, Baltimore, MD), Robert Wise (Johns Hopkins University, Baltimore, MD), Patrick Breysse (Johns Hopkins University, Baltimore, MD), D’Ann Williams (Johns Hopkins University, Baltimore, MD).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elmasri, M., Romero, K.M., Gilman, R.H. et al. Longitudinal Assessment of High Versus Low Levels of Fractional Exhaled Nitric Oxide Among Children with Asthma and Atopy. Lung 192, 305–312 (2014). https://doi.org/10.1007/s00408-013-9551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9551-8