Abstract
Purposes
To investigate the effect and safety of ovarian tissue cryopreservation (OTC) for fertility preservation in female patients with hematological diseases.
Methods
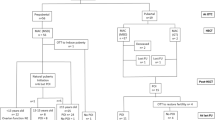
We designed a retrospective study. The clinical data of patients with hematological diseases undergoing OTC admitted to Peking University People’s Hospital from April 2017 to January 2023 were analyzed and summarized.
Results
A total of 24 patients were included in the study, including 19 patients with malignant hematological diseases and 5 patients with non-malignant hematological diseases. The former included 14 patients with acute leukemia, 1 patient with chronic leukemia, and 4 patients with myelodysplastic syndrome, while the latter 5 patients were aplastic anemia (AA). 16 patients had received chemotherapy before OTC. The average age of 24 patients was 22.80 ± 6.81 years. The average anti-Mullerian hormone (AMH) was 1.97 ± 2.12 ng/mL, and the average follicle-stimulating hormone (FSH) was 7.01 ± 4.24 IU/L in examination before OTC. FSH was greater than 10.0 IU/L in 4 cases. The pre-OTC laboratory tests showed that the average white blood cell (WBC) count was (3.33 ± 1.35) × 109/L, the average hemoglobin was 91.42 ± 22.84 g/L, and the average platelet was (147.38 ± 114.46) × 109/L. After injection of recombinant human granulocyte colony-stimulating factor (rhG-CSF), blood transfusion, and iron supplementation in pre-OTC treatment, the average WBC count was (4.91 ± 3.07) × 109/L, the average hemoglobin was 98.67 ± 15.43 g/L, and the average platelet was (156.38 ± 103.22) × 109/L.
Of the 24 patients, 22 underwent laparoscopic bilateral partial oophorectomy and oophoroplasty, and 2 underwent laparoscopic unilateral oophorectomy. The average duration of OTC was 59.54 ± 17.58 min, and the average blood loss was 32.1 ± 41.6 mL. The maximum blood loss was 200 mL.
There was no significant difference in WBC count and hemoglobin concentration after OTC compared to pre-OTC period. Only the platelet count after OTC surgery was significantly different from that before surgery ([134.54 ± 80.84 vs. 156.38 ± 103.22] × 109/L, p < 0.05). None of the 24 patients had serious complications after OTC. 2 patients had mild infection symptoms, but both recovered well. 23 patients underwent hematopoietic stem cell transplantation (HSCT) after OTC. The median and interquartile range from OTC to the pretreatment of HSCT was 33 (57) days, and the median and interquartile range from OTC to HSCT was 41 (57) days. Seven of them began pretreatment of HSCT within 20 days and began HSCT within 30 days after OTC. All patients were followed up. Of the 23 patients who underwent HSCT after surgery, 22 presented with amenorrhea and 1 with scanty menstrual episodes. Seven patients underwent hormone replacement therapy (HRT) after HSCT. A patient with AA underwent ovarian tissue transplantation (OTT) 3 years after HSCT and resumed regular menstruation 6 months after OTT.
Conclusions
Ovarian tissue cryopreservation has a promising future in fertility protection in patients with hematological diseases. However, patients with hematological malignancies often have received gonadotoxic therapy before OTC, which may be accompanied by myelosuppression while patients with non-malignant hematological diseases often present with severe hemocytopenia. So perioperative complete blood count of patients should be paid attention to. There was no significant difference in the WBC count and hemoglobin concentration in patients with hematological diseases before and after OTC surgery, and the platelet count decreased slightly within the normal range. Infection is the most common post-OTC complication, and HSCT pretreatment can be accepted as early as the 10th day after OTC. OTC has no adverse effects on patients with hematological diseases and does not delay HSCT treatment. For young patients with hematological diseases, OTC is an effective method of fertility preservation.


Similar content being viewed by others
Data availability
Data will be available on request.
Abbreviations
- MDS:
-
Myelodysplastic syndromes
- AA:
-
Aplastic anemia
- CAMT:
-
Congenital amegakaryocytic thrombocytopenia
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- HSCT:
-
Hematopoietic stem cell transplantation
- OTC:
-
Ovarian tissue cryopreservation
- CAR-T:
-
Chimeric antigen receptor T-cell immunotherapy
- CMML:
-
Chronic myelomonocytic leukemia
- SPSS:
-
Specific prognostic scoring system
- HPO:
-
Hypothalamic-pituitary-ovarian
- AMH:
-
Anti-mullerian hormone
- rhG-CSF:
-
Recombinant human granulocyte colony stimulating factor
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- OTT:
-
Ovarian tissue transplantation
- WBC:
-
White blood cell
References
Sung H et al (2021) Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for cancers in 185 countries. A Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Ni X et al (2022) Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet 400(10357):1020–1032. https://doi.org/10.1016/S0140-6736(22)01541-0
Loren AW, Senapati S (2019) Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 134(9):746–760. https://doi.org/10.1182/blood.2018846790
Scott BL et al (2017) Myeloablative versus reduced—intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 35(11):1154–1161. https://doi.org/10.1200/JCO.2016.70.7091
Wallace WHBP et al (2014) Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol 15(10):1129–1136. https://doi.org/10.1016/S1470-2045(14)70334-1
Martinez F (2017) Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril 108(3):407-415.e11. https://doi.org/10.1093/humrep/dex218
International Society for Gynecological Endocrinology (2018) China gynecological endocrinology section and consensus expert, chinese expert consensus on ovarian tissue cryopreservation and transplantation. Chinese J Clinic 46(4):496–500. https://doi.org/10.3969/j.issn.2095-8552.2018.04.042
Beijing Maternity Hospital of Capital Medical University (2023) Professional committee on fertility protection and preservation, chinese society for the promotion of human health science and technology (CSPHST), Ruan xy, specification for ovarian tissue cryopreservation and transplantation. Chinese Family Med 26(23):2836–2841. https://doi.org/10.12114/j.issn.1007-9572.2023.0237
Ruan X (2018) Chinese society of gynecological endocrinology affiliated to the international society of gynecological endocrinology guideline for ovarian tissue cryopreservation and transplantation. Gynecol Endocrinol 34(12):1005–1010. https://doi.org/10.1080/09513590.2018.1488957
Loren AW et al (2013) Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 31(19):2500–2510. https://doi.org/10.1200/JCO.2013.49.2678
Alson S et al (2018) Anti-mullerian hormone levels are associated with live birth rates in ART, but the predictive ability of anti-mullerian hormone is modest. Eur J Obstet Gynecol Reprod Biol 225:199–204. https://doi.org/10.1016/j.ejogrb.2018.04.039
Subgroup M (2023) Chinese society of obstetrics and gynecology, Chinese medical association, consensus of clinical diagnosis and treatment of premature ovarian insufficiency (2023). Chinese J Obstet Gynecol 58(10):721–728. https://doi.org/10.3760/cma.j.cn112141-20230316-00122
Reynolds AC, McKenzie LJ (2023) Cancer treatment—related ovarian dysfunction in women of childbearing potential: management and fertility preservation options. J Clin Oncol 41(12):2281–2292. https://doi.org/10.1200/JCO.22.01885
Bedoschi G, Navarro PA, Oktay K (2016) Chemotherapy—induced damage to ovary: mechanisms and clinical impact. Future Oncol 12(20):2333–2344. https://doi.org/10.2217/fon-2016-0176
Mauri D et al (2020) Chemotherapy associated ovarian failure front endocrinol (Lausanne) 11:572388. https://doi.org/10.3389/fendo.2020.572388
Nurmio M et al (2022) Effect of previous alkylating agent exposure on follicle numbers in cryopreserved prepubertal and young adult ovarian tissue after long-term xenografting. Cancers 14(2):399. https://doi.org/10.3390/cancers14020399
Dolmans MM et al (2016) Evaluation of minimal disseminated disease in cryopreserved ovarian tissue from bone and soft tissue sarcoma patients. Hum Reprod 31(10):2292–2302. https://doi.org/10.1093/humrep/dew193
Lambertini M et al (2016) Cancer and fertility preservation: International recommendations from an expert meeting. BMC Med 14(1):1–1. https://doi.org/10.1186/s12916-015-0545-7
Takae S et al (2021) Surgical management of unilateral oophorectomy for ovarian tissue cryopreservation in high-risk children and adolescents with varied backgrounds. Pediatr Surg Int 37(8):1021–1029. https://doi.org/10.1007/s00383-021-04900-7
Jabbour E et al (2023) The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J Hematol Oncol 16(1):22–22. https://doi.org/10.1186/s13045-023-01409-5
McGrath S et al (2020) Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 29(9):2520–2537. https://doi.org/10.1177/0962280219889080
Oktay KMDF, Oktem OMD (2010) Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril 93(3):762–768. https://doi.org/10.1016/j.fertnstert.2008.10.006
Corkum KS, Laronda MM, Rowell EE (2017) A review of reported surgical techniques in fertility preservation for prepubertal and adolescent females facing a fertility threatening diagnosis or treatment. The American journal of surgery 214(4):695–700. https://doi.org/10.1016/j.amjsurg.2017.06.013
Beckmann MW et al (2018) Fertility protection: complications of surgery and results of removal and transplantation of ovarian tissue. Reprod Biomed Online 36(2):188–196. https://doi.org/10.1016/j.rbmo.2017.10.109
Puy V et al (2023) Ovarian tissue cryopreservation can be combined simultaneously with oocyte retrieval after controlled ovarian hyperstimulation. Hum Reprod 38(5):860–871. https://doi.org/10.1093/humrep/dead041
Ruan X (2023) Guidelines for the clinical application of ovarian tissue cryopreservation transplantation for the prevention and treatment of medically induced early onset ovarian insufficiency. J Capi Medi Univ 44(05):695–703. https://doi.org/10.3969/j.issn.1006-7795.2023.05.001
Ruan X (2022) Clinical application of ovarian tissue cryopreservation and transplantation for fertility preservation. Chinese J Prac Gynecol and Obsts 38(06):599–604. https://doi.org/10.19538/j.fk2022060107
Zver T et al (2022) Minimal residual disease detection by multicolor flow cytometry in cryopreserved ovarian tissue from leukemia patients. J Ovarian Res 15(1):9. https://doi.org/10.1186/s13048-021-00936-4
Shirai R et al (2023) Minimal residual disease detection by mutation-specific droplet digital PCR for leukemia/lymphoma. Int J Hematol 117(6):910–918. https://doi.org/10.1007/s12185-023-03566-2
Funding
This work was supported by “Natural Science Foundation of Beijing Municipality” (No: 7222206).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking University People’s Hospital Ethics Committee (2024PHB042-001).
Consent to participate
This study is a retrospective study and informed consent had been waived by Ethics Committee of Peking University People’s Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YL., Zhai, QJ., Wang, ZH. et al. A retrospective study of ovarian tissue cryopreservation in female patients with hematological diseases for fertility preservation. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07484-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-024-07484-4