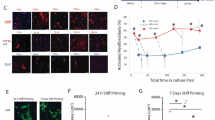
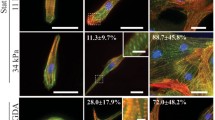
Abstract
The mechanical environment of the myocardium has a potent effect on cardiomyocyte form and function, yet an understanding of the cardiomyocyte responses to extracellular stiffening remains incomplete. We therefore employed a cell culture substrate with tunable stiffness to define the cardiomyocyte responses to clinically relevant stiffness increments in the absence of cell–cell interactions. When cultured on substrates magnetically actuated to mimic the stiffness of diseased myocardium, isolated rat adult cardiomyocytes exhibited a time-dependent reduction of sarcomere shortening, characterized by slowed contraction and relaxation velocity, and alterations of the calcium transient. Cardiomyocytes cultured on stiff substrates developed increases in viscoelasticity and microtubule detyrosination in association with early increases in the α-tubulin detyrosinating enzyme vasohibin-2 (Vash2). We found that knockdown of Vash2 was sufficient to preserve contractile performance as well as calcium transient properties in the presence of extracellular substrate stiffening. Orthogonal prevention of detyrosination by overexpression of tubulin tyrosine ligase (TTL) was also able to preserve contractility and calcium homeostasis. These data demonstrate that a pathologic increment of extracellular stiffness induces early, cell-autonomous remodeling of adult cardiomyocytes that is dependent on detyrosination of α-tubulin.









Similar content being viewed by others
References
Agnetti G, Halperin VL, Kirk JA, Chakir K, Guo Y, Lund L, Nicolini F, Gherli T, Guarnieri C, Caldarera CM, Tomaselli GF, Kass DA, Van Eyk JE (2014) Desmin modifications associate with amyloid-like oligomers deposition in heart failure. Cardiovasc Res 102:24–34
Bayomy AF, Bauer M, Qiu Y, Liao R (2012) Regeneration in heart disease-Is ECM the key? Life Sci 91:823–827
Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL (2006) Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290:2196
Boothe SD, Myers JD, Pok S, Sun J, Xi Y, Nieto RM, Cheng J, Jacot JG (2016) The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem Biophys 74:527–535
Caporizzo MA, Chen CY, Prosser BL (2019) Cardiac microtubules in health and heart disease. Exp Biol Med (Maywood) 244:1255–1272
Caporizzo MA, Chen CY, Bedi K, Margulies KB, Prosser BL (2020) Microtubules increase diastolic stiffness in failing human cardiomyocytes and myocardium. Circulation 141:902–915
Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB, Prosser BL (2018) Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med 24:1225–1233
Chen CY, Salomon AK, Caporizzo MA, Curry S, Kelly NA, Bedi K, Bogush AI, Kramer E, Schlossarek S, Janiak P, Moutin MJ, Carrier L, Margulies KB, Prosser BL (2020) Depletion of vasohibin 1 speeds contraction and relaxation in failing human cardiomyocytes. Circ Res 127:e14–e27
Cheng G, Zile MR, Takahashi M, Baicu CF, Bonnema DD, Cabral F, Menick DR, Cooper G (2008) A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am J Physiol Heart Circ Physiol 294:2231
Chinnakkannu P, Samanna V, Cheng G, Ablonczy Z, Baicu CF, Bethard JR, Menick DR, Kuppuswamy D, Cooper G (2010) Site-specific microtubule-associated protein 4 dephosphorylation causes microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 285:21837–21848
Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH (1995) Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91:161–170
Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, Margulies KB (2019) Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces 11:20603–20614
Crocini C, Walker CJ, Anseth KS, Leinwand LA (2020) Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog Biophys Mol Biol 154:71–79
Galie PA, Khalid N, Carnahan KE, Westfall MV, Stegemann JP (2013) Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc Pathol 22:219–227
Gutierrez C, Blanchard DG (2004) Diastolic heart failure: challenges of diagnosis and treatment. Am Fam Physician 69:2609–2616
Ishibashi Y, Tsutsui H, Yamamoto S, Takahashi M, Imanaka-Yoshida K, Yoshida T, Urabe Y, Sugimachi M, Takeshita A (1996) Role of microtubules in myocyte contractile dysfunction during cardiac hypertrophy in the rat. Am J Physiol 271:1978
Jacot JG, McCulloch AD, Omens JH (2008) Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95:3479–3487
Janmey PA, Miller RT (2011) Mechanisms of mechanical signaling in development and disease. J Cell Sci 124:9–18
Janmey PA, Fletcher DA, Reinhart-King CA (2020) Stiffness sensing by cells. Physiol Rev 100:695–724
Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen Y, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, Chen-Izu Y (2014) Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal 7:ra27. https://doi.org/10.1126/scisignal.2005046
Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, Becerra N, Harki DA, Martin SS, Raiteri R, Prosser BL, Ward CW (2015) Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 6:8526
Koshy SK, Reddy HK, Shukla HH (2003) Collagen cross-linking: new dimension to cardiac remodeling. Cardiovasc Res 57:594–598
Krenning G, Zeisberg EM, Kalluri R (2010) The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225:631–637
Kronenbitter A, Funk F, Hackert K, Gorreßen S, Glaser D, Boknik P, Poschmann G, Stühler K, Isić M, Krüger M, Schmitt JP (2018) Impaired Ca2+ cycling of nonischemic myocytes contributes to sarcomere dysfunction early after myocardial infarction. J Mol Cell Cardiol 119:28–39. https://doi.org/10.1016/j.yjmcc.2018.04.004
Loescher CM, Hobbach AJ, Linke WA. (2021) Titin (TTN): from molecule to modifications, mechanics and medical significance. Cardiovasc Res. cvab328 [pii]
McCain ML, Parker KK (2011) Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch 462:89–104
Pessot G, Schumann M, Gundermann T, Odenbach S, Lowen H, Menzel AM (2018) Tunable dynamic moduli of magnetic elastomers: from characterization by x-ray micro-computed tomography to mesoscopic modeling. J Phys Condens Matter. https://doi.org/10.1088/1361-648X/aaaeaa
Phyo SA, Uchida K, Chen CY, Caporizzo MA, Bedi K, Griffin J, Margulies K, Prosser BL (2022) Transcriptional, post-transcriptional, and post-translational mechanisms rewrite the tubulin code during cardiac hypertrophy and failure. Front Cell Dev Biol. https://doi.org/10.1101/2022.01.24.477567
Poh Y, Chowdhury F, Tanaka TS, Wang N (2010) Embryonic stem cells do not stiffen on rigid substrates. Biophys J 99:19. https://doi.org/10.1016/j.bpj.2010.04.057
Robison P, Prosser BL (2017) Microtubule mechanics in the working myocyte. J Physiol 595:3931–3937
Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL (2016) Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. https://doi.org/10.1126/science.aaf0659
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3:7–11
Setterberg IE, Le C, Frisk M, Perdreau-Dahl H, Li J, Louch WE (2021) The physiology and pathophysiology of T-tubules in the heart. Front Physiol 12:718404. https://doi.org/10.3389/fphys.2021.718404
Sumita Yoshikawa W, Nakamura K, Miura D, Shimizu J, Hashimoto K, Kataoka N, Toyota H, Okuyama H, Miyoshi T, Morita H, Fukushima Kusano K, Matsuo T, Takaki M, Kajiya F, Yagi N, Ohe T, Ito H (2013) Increased passive stiffness of cardiomyocytes in the transverse direction and residual actin and myosin cross-bridge formation in hypertrophied rat hearts induced by chronic beta-adrenergic stimulation. Circ J 77:741–748
Tagawa H (1996) Cytoskeletal role in the contractile dysfunction of cardiocytes from hypertrophied and failing right ventricular myocardium. Proc Assoc Am Physicians 108:218–229
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118:1021–1040
Tsutsui H, Ishihara K, Cooper G (1993) Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 260:682–687
van Deel ED, Najafi A, Fontoura D, Valent E, Goebel M, Kardux K, Falcao-Pires I, van der Velden J (2017) In vitro model to study the effects of matrix stiffening on Ca(2+) handling and myofilament function in isolated adult rat cardiomyocytes. J Physiol 595:4597–4610
van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ (2012) Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 9:293–302
Wheelwright M, Win Z, Mikkila JL, Amen KY, Alford PW, Metzger JM (2018) Investigation of human iPSC-derived cardiac myocyte functional maturation by single cell traction force microscopy. PLoS One 13:e0194909
Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M (2002) Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res 55:76–82
Yao J, Sun Y, Wang Y, Fu Q, Xiong Z, Liu Y (2018) Magnet-induced aligning magnetorheological elastomer based on ultra-soft matrix. Composites Sci Technol 162:170–179. https://doi.org/10.1016/j.compscitech.2018.04.036
Young JL, Kretchmer K, Ondeck MG, Zambon AC, Engler AJ (2014) Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci Rep 4:6425
Yu X, Chen X, Amrute-Nayak M, Allgeyer E, Zhao A, Chenoweth H, Clement M, Harrison J, Doreth C, Sirinakis G, Krieg T, Zhou H, Huang H, Tokuraku K, Johnston DS, Mallat Z, Li X (2021) MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 594:560–565. https://doi.org/10.1038/s41586-021-03573-5
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350:1953–1959
Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM (2015) Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131:1247–1259. https://doi.org/10.1161/CIRCULATIONAHA.114.013215
Zile MR, Koide M, Sato H, Ishiguro Y, Conrad CH, Buckley JM, Morgan JP, Cooper G (1999) Role of microtubules in the contractile dysfunction of hypertrophied myocardium. J Am Coll Cardiol 33:250–260
Funding
This research was supported by funding from NIH/NHLBI R01-HL149891-01 to K.B.M and B.L.P., R01-HL133080 to B.L.P, a Leducq Fondation award TNE ID#: 673168 to B.L.P and K.B.M., Gund Family Fund support to K.B.M., and AHA CDA 856504 to M.A.C.
Author information
Authors and Affiliations
Contributions
AV, MAC, BLP, and KBM developed the research strategy. AV, MAC, EAC BLP, and KBM participated in the design of experiments. AV, MAC, EAC, JB, QM and CEL performed the experiments. All authors participated in the writing and review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Drs. Prosser and Margulies report significant financial interests: invention disclosure/patent; inventor: US patent application No.15/959,181 USA 2018, composition and methods for improving heart function and treating heart failure.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vite, A., Caporizzo, M.A., Corbin, E.A. et al. Extracellular stiffness induces contractile dysfunction in adult cardiomyocytes via cell-autonomous and microtubule-dependent mechanisms. Basic Res Cardiol 117, 41 (2022). https://doi.org/10.1007/s00395-022-00952-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00952-5