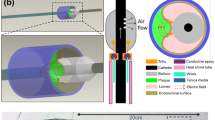
Abstract
Interventional techniques are necessary, which allow the characterization of intravascular pathological processes. Electric impedance spectroscopy (EIS) can provide cellular information of biological tissue. We tested the feasibility of intravascular EIS by using a new impedance catheter system with integrated microelectrodes in an experimental animal model. Eighteen stents were implanted into the iliac arteries of female New Zealand White rabbits (n = 11) to induce intimal proliferation. After 14, 28 and 56 days the electric impedance was measured inside and outside of the stented arterial segments by using a balloon catheter with four integrated microelectrodes. The impedance was recorded at a frequency ranging from 1 Hz to 1 MHz. After the measurements, the stents were explanted and histomorphometry was performed. The impedance inside and outside the stent was analysed and compared with the histomorphometric data.
Fourteen (n = 6), 28 (n = 5) and 56 (n = 6) days after stent implantation the difference of the electrical impedance between the native and the stented iliac artery segment increased from –924 ± 715 Ohm to 3689 ± 1385 Ohm (14 days vs. 28 days; p < 0.05) and 8637 ± 2881 Ohm (14 days vs. 56 days; p < 0.05), respectively. The increase of the electrical impedance corresponded to an increased neointimal proliferation in the stented arterial segment of 3.6% ± 0.7% after 14 days, 8.4% ± 4.8% after 28 days (14 days vs. 28 days; p < 0.05) and 10.0% ± 4.1% after 56 days (14 days vs. 56 days; p < 0.01).
Intravascular EIS can be performed by a balloon catheter with integrated microelectrodes and allows the detection of neointimal proliferation after stent implantation.
Similar content being viewed by others
References
Fuster V, Badimon L, Badiman JJ, Chesobro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 326:242–250
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Rössig l, Dimmeler S, Zeiher AM (2001) Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol 96:11–22
Little WC, Constantinescu M, Applegate RJ et al. (1988) Can coronary angiography predict the site of subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 78:1157–1166
De Korte CL, Pasterkamp G, van der Steen AF, Woutman HA, Bom N (2000) Characterization of plaque components with intravascular ultrasound elastography in human femoral and coronary arteries. Circulation 102:617–623
de Korte CL, Sierevogel MJ, Mastik F et al. (2002) Identi.cation of atherosclerotic plaque components with intravascular ultrasound elastography in vivo: a Yucatan pig study. Circulation 105:1627–1630
Nair A, Kuban BD, Tuzcu EM Schoenhagen P, Nissen SE, Vince DG (2002) Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106:2200–2206
Stahr PM, Hofflinghaus T, Voigtlander T et al. (2002) Discrimination of early/intermediate and advanced/complicated coronary plaque types by radiofrequency intravascular ultrasound analysis. Am J Cardiol 90:19–23
Verheye S, De Meyer GR, Van Langenhove G, Knaapen MW, Kockx MM (2002) In vivo temperature heterogeneity of atherosclerotic plaques is determined by plaque composition. Circulation 105:1596–1601
Stefanadis C, Diamantopoulos L, Vlachopoulos C et al. (1999) Thermal heterogeneity within atherosclerotic coronary arteries detected in vivo: A new method of detection by application of a special thermography catheter. Circulation 99:1965–1971
Stefanadis C, Toutouzas K, Tsiamis E et al. (2001) Increased local temperature in human coronary atherosclerotic plaques: an independent predictor of clinical outcome in patients undergoing a percutaneous coronary intervention. J Am Coll Cardiol 37:1277–1283
Foster KR, Schwan HP (1989) Dielectric properties of tissue and biological material: a critical review. Crit Rev Biomed Eng 17:25–104
Schwan, HP (1993) Mechanisms responsible for electric properties of tissues and cell suspensions. Med Prog Technol 19:163–165
Brown BH, Tidy JA, Boston K, Blackett AD, Smallwood RH, Sharp F (2000) Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 355:892–895
Wilkinson BA, Smallwood RH, Keshtar A, Lee JA, Hamdy FC (2002) Electrical impedance spectroscopy and the diagnosis of bladder pathology: a pilot study. J Urol 168:1563–1567
Stieglitz T, Beutel H, Schüttler M et al. (2000) Micromachined, polyimide-based devices for flexible neural interfaces. Biomedical Microdevices 4:283–294
Steendijk P, Mur G, van der Welde ET, Baan J (1993) The four-electrode resistivity technique in anisotropic media: Theoretical analysis and application on myocardial tissue in vivo. IEEE Trans Biomed Eng 40:1138–1148
Tsai JZ, Cao H, Tungjitkusolmun S, Woo EJ, Vorperian VR, Webster JG (2000) Dependence of apparent resistance of four-electrode probes on insertion depth. IEEE Trans Biomed Eng 47:41–48
Ivorra A, Gomez R, Noguera N, Villa R, Sola A, Palacios L, Hotter G, Aguilo J (2003) Minimally invasive silicon probe for electrical impedance measurements in small animals. Biosens Bioelectron 15:391–399
Slager CJ, Phaff AC, Essed CE, Bom N, Schuurbiers JC, Serruys PW (1992) Electrical impedance of layered atherosclerotic plaques on human aortas. IEEE Trans Biomed Eng 39:411–419
Konings MK, Mali WP, Viergever MA (1997) Development of an intravascular impedance catheter for detection of fatty lesions in arteries. IEEE Trans Med Imag 16:439–446
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Süselbeck and Thielecke equally contributed to the work presented in this manuscript.
This study was presented in part at the American College of Cardiology Scientific Sessions, Chicago, March 30 – April 2, 2003.
Rights and permissions
About this article
Cite this article
Süselbeck, T., Thielecke, H., Weinschenk, I. et al. In vivo intravascular electric impedance spectroscopy using a new catheter with integrated microelectrodes. Basic Res Cardiol 100, 28–34 (2005). https://doi.org/10.1007/s00395-004-0501-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-004-0501-8