Abstract
Purpose
Withdrawal of thiopurines after remission is associated with an increased risk of relapse in patients with inflammatory bowel disease (IBD). However, long-term data on thiopurine withdrawal is limited, especially from developing countries where the cost of long-term therapy poses a significant burden on patients.
Methods
Patients with IBD on thiopurine monotherapy for ≥ 4 months, who stopped thiopurines while in clinical remission and were not on any other immunomodulator or biologics at the time of withdrawal, were included in this retrospective analysis.
Results
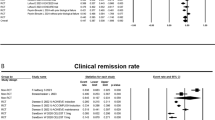
Among 1093 patients with IBD on thiopurine monotherapy, 461 patients stopped thiopurine due to various reasons. Among these, 218 (ulcerative colitis (UC) = 179; Crohn’s disease (CD) = 39) patients were in clinical remission and were continued on mesalamine. Overall, 36.7% (n = 80) relapsed after a median duration of 20 months (IQR: 9–49). Relapse rate was higher in UC than CD (39.7% vs 23%, p = 0.055). Cumulative probabilities of relapse were 17%, 34%, and 44% at the end of 1, 3, and 5 years, respectively. The relapse rate at 5 years was significantly lower in patients who had stopped azathioprine after 4 years of therapy (31% vs 54%, p = 0.007). On multi-variate cox regression analysis, male sex [HR: 1.6(1.0–2.6), p = 0.02] and short duration of therapy with thiopurines [HR: 1.02 (1.01–1.02), p = 0.004] before withdrawal were associated with increased risk of relapse.
Conclusion
Approximately 50% patients with IBD in remission would relapse after 5 years of thiopurine withdrawal. Male sex and shorter treatment duration predict relapse. Treatment should be continued in patients who tolerate and maintain remission on long-term thiopurine.





Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kaplan GG, Windsor JW (2021) The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 18(1):56–66. https://doi.org/10.1038/s41575-020-00360-x
Alatab S, Sepanlou SG, Ikuta K et al (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5(1):17–30. https://doi.org/10.1016/S2468-1253(19)30333-4
Kedia S, Ahuja V (2017) Epidemiology of inflammatory bowel disease in India: the great shift east. Inflamm Intest Dis 2(2):102–115. https://doi.org/10.1159/000465522
Gomollón F, Dignass A, Annese V et al (2017) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 11(1):3–25. https://doi.org/10.1093/ecco-jcc/jjw168
Magro F, Gionchetti P, Eliakim R et al (2017) Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. Journal of Crohn’s Colitis. 11(6). https://doi.org/10.1093/ecco-jcc/jjx008
Jewell DP, Truelove SC (1974) Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J 4(5945):627–630. https://doi.org/10.1136/bmj.4.5945.627
Sood A, Midha V, Sood N, Kaushal V (2000) Role of azathioprine in severe ulcerative colitis: one-year, placebo-controlled, randomized trial. Indian J Gastroenterol 19(1):14–16
Gisbert JP, Linares PM, Mcnicholl AG, Maté J, Gomollón F (2009) Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther 30(2):126–137. https://doi.org/10.1111/j.1365-2036.2009.04023.x
Stournaras E, Qian W, Pappas A et al (2021) Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in Crohn’s disease: long-term outcomes for 11 928 patients in the UK inflammatory bowel disease bioresource. Gut 70(4):677–686. https://doi.org/10.1136/gutjnl-2019-320185
Raine T, Bonovas S, Burisch J et al (2021) ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn’s Colitis. Published Online Oct 12 2021:jjab178. https://doi.org/10.1093/ecco-jcc/jjab178
Torres J, Organisation [ECCO on behalf of the EC and C, Bonovas S et al (2020) ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 14(1):4–22. https://doi.org/10.1093/ecco-jcc/jjz180
Sandborn WJ, Su C, Sands BE et al (2017) Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 376(18):1723–1736. https://doi.org/10.1056/NEJMoa1606910
1 Recommendations | Tofacitinib for moderately to severely active ulcerative colitis | Guidance | NICE. Accessed June 27, 2022. https://www.nice.org.uk/guidance/ta547/chapter/1-Recommendations
Huang SZ, Liu ZC, Liao WX et al (2019) Risk of skin cancers in thiopurines-treated and thiopurines-untreated patients with inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 34(3):507–516. https://doi.org/10.1111/jgh.14533
Vos ACW, Bakkal N, Minnee RC et al (2011) Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis 17(9):1837–1845. https://doi.org/10.1002/ibd.21582
O’Donoghue DP, Dawson AM, Powell-Tuck J, Bown RL, Lennard-Jones JE (1978) Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet 2(8097):955–957. https://doi.org/10.1016/s0140-6736(78)92524-2
Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J (2004) Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: increased relapse rate the following year. Aliment Pharmacol Ther 19(11):1147–1152. https://doi.org/10.1111/j.1365-2036.2004.01944.x
Hawthorne AB, Logan RF, Hawkey CJ et al (1992) Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 305(6844):20–22. https://doi.org/10.1136/bmj.305.6844.20
Lémann M, Mary JY, Colombel JF et al (2005) A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology 128(7):1812–1818. https://doi.org/10.1053/j.gastro.2005.03.031
Wenzl HH, Primas C, Novacek G et al (2015) Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig Dis Sci 60(5):1414–1423. https://doi.org/10.1007/s10620-014-3419-5
Kennedy NA, Kalla R, Warner B et al (2014) Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther 40(11–12):1313–1323. https://doi.org/10.1111/apt.12980
Cassinotti A, Corona A, Duca P et al (2021) Noninvasive monitoring after azathioprine withdrawal in patients with inflammatory bowel disease in deep remission. Clin Gastroenterol Hepatol 19(11):2293-2301.e1. https://doi.org/10.1016/j.cgh.2021.06.014
Cassinotti A, Actis GC, Duca P et al (2009) Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. Am J Gastroenterol 104(11):2760–2767. https://doi.org/10.1038/ajg.2009.410
Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J (2015) Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 149(7):1716–1730. https://doi.org/10.1053/j.gastro.2015.08.055
Prefontaine E, MacDonald JK, Sutherland LR (2010) Azathioprine or 6‐mercaptopurine for induction of remission in Crohn’s disease. Cochrane Data Syst Rev (6). https://doi.org/10.1002/14651858.CD000545.pub3
Su C, Lichtenstein GR (2004) Treatment of inflammatory bowel disease with azathioprine and 6-mercaptopurine. Gastroenterol Clin North Am 33(2):209–234, viii. https://doi.org/10.1016/j.gtc.2004.02.004
Verstockt B, Boets L, Sabino J, Vermeire S, Ferrante M (2021) Thiopurine monotherapy has a limited place in treatment of patients with mild-to-moderate Crohn’s disease. Gut 70(7):1416–1418. https://doi.org/10.1136/gutjnl-2020-322646
Harbord M, Eliakim R, Bettenworth D et al (2017) Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 11(7):769–784. https://doi.org/10.1093/ecco-jcc/jjx009
van der Heide F, Dijkstra A, Weersma RK et al (2009) Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 15(8):1199–1207. https://doi.org/10.1002/ibd.20884
Bouhnik Y, Lémann M, Mary JY et al (1996) Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet 347(8996):215–219. https://doi.org/10.1016/s0140-6736(96)90402-x
Fraser AG, Orchard TR, Jewell DP (2002) The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 50(4):485–489. https://doi.org/10.1136/gut.50.4.485
Qian X, Wang T, Shen J, Ran Z (2018) Low dose of azathioprine is effective to induce and maintain remission in active Crohn disease: a prospective observational study. Medicine 97(34):e11814. https://doi.org/10.1097/MD.0000000000011814
Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T (2003) Low-dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol 38(8):740–746. https://doi.org/10.1007/s00535-003-1139-2
Shi HY, Chan FKL, Leung WK et al (2016) Low-dose azathioprine is effective in maintaining remission in steroid-dependent ulcerative colitis: results from a territory-wide Chinese population-based IBD registry. Therap Adv Gastroenterol 9(4):449–456. https://doi.org/10.1177/1756283X16643509
Higgins PDR, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM (2005) Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 54(6):782–788. https://doi.org/10.1136/gut.2004.056358
Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR (2003) Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol 38(2):164–171. https://doi.org/10.1080/00365520310000654
Doherty G, Katsanos KH, Burisch J et al (2018) European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘Exit Strategies’] in inflammatory bowel disease. Journal of Crohn’s and Colitis 12(1):17–31. https://doi.org/10.1093/ecco-jcc/jjx101
Ng SC, Tang W, Ching JY et al (2013) Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 145(1):158-165.e2. https://doi.org/10.1053/j.gastro.2013.04.007
Funding
Indian Council of Medical Research- Center for Advanced Research in Intestinal diseases.
Author information
Authors and Affiliations
Contributions
Mukesh Kumar Ranjan: acquisition of data, interpretation of data and drafting the article, revising it critically for important intellectual content, and final approval of manuscript. Sudheer K. Vuyyuru: analysis with interpretation of data and drafting the article, revising it critically for important intellectual content, and final approval of manuscript. Bhaskar Kante: drafting the article, revising it critically for important intellectual content, and final approval of manuscript. Peeyush Kumar: acquisition of data, critical revision of manuscript for important intellectual content, and final approval of manuscript. Sandeep Mundhra: acquisition of data, critical revision of manuscript for important intellectual content, and final approval of manuscript. Rithvik Golla: acquisition of data, critical revision of manuscript for important intellectual content, and final approval of manuscript. Raju Sharma: drafting the article and revising it critically for important intellectual content and final approval of manuscript. Peush Sahni: drafting the article and revising it critically for important intellectual content, final approval of manuscript. Prasenjit Das: drafting the article and revising it critically for important intellectual content and final approval of manuscript. Govind Makharia: drafting the article and revising it critically for important intellectual content and final approval of manuscript. Saurabh Kedia: interpretation of data and drafting the article, revising it critically for important intellectual content, and final approval of manuscript. Vineet Ahuja: study concept, study design, study supervision, interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan, M.K., Vuyyuru, S.K., Kante, B. et al. Relapse rates after withdrawal of thiopurines in patients with inflammatory bowel disease. Int J Colorectal Dis 37, 1817–1826 (2022). https://doi.org/10.1007/s00384-022-04216-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04216-5