Abstract
Purpose
Although some biomarkers of hepatic progenitor cells have been reported to be involved in the liver fibrosis in patients with biliary atresia (BA), however, research still shows conflicting results. Therefore, we investigated the effect of Alu-mediated p21 transcriptional regulator (APTR), fibroblast growth factor-inducible 14 (Fn14) and CD133 expressions on liver fibrosis in Indonesian BA patients.
Methods
Nineteen liver samples from BA patients and 9 liver specimens from non-BA controls were obtained. The expressions of APTR, Fn14 and CD133 were analyzed by quantitative real-time polymerase chain reaction (qPCR).
Results
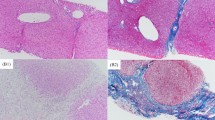
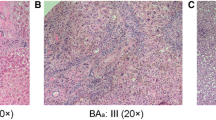
APTR expression was strongly up-regulated (1.5-fold) in liver BA specimens compared to liver controls (ΔCT 3.2 ± 0.6 vs 3.8 ± 0.51; p = 0.028). Moreover, Fn14 and CD133 expressions were similar in the BA and control groups (ΔCT 2.7 ± 1.3 vs. 1.4 ± 1.6, p = 0.07; and 12.0 ± 3.7 vs. 11.78 ± 2.30, p = 0.88, respectively). Intriguingly, CD133 expression was strongly related with the survival of BA patients (p = 0.0061), but not with age at Kasai procedure (p = 0.36) and the presence of cirrhosis (p = 0.77).
Conclusion
We present the first study of aberrant APTR expressions in the liver of BA infants which might contribute to liver fibrogenesis in BA infants.


Similar content being viewed by others
References
Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH (2007) Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 46:566–581
Lakshminarayanan B, Davenport M (2016) Biliary atresia: a comprehensive review. J Autoimmun 73:1–9
Sundaram SS, Mack CL, Feldman AG, Sokol RJ (2017) Biliary atresia: indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl 23:96–109
Wada H, Muraji T, Yokoi A et al (2007) Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: a regional survey of over 20 years. J Pediatr Surg 42:2090–2092
Chiu CY, Chen PH, Chan CF, Chang MH, Wu TC, Taiwan Infant Stool Color Card Study Group et al (2013) Biliary atresia in preterm infants in Taiwan: a nationwide survey. J. Pediatr 163:100–103
Gunadi, Gunawan TA, Widiyanto G, Yuanita A, Mulyani NS, Makhmudi A (2018) Liver transplant score for prediction of biliary atresia patients' survival following Kasai procedure. BMC Res Notes 11:381
Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B et al (2013) Improving outcomes of biliary atresia: French national series 1986–2009. J Hepatol 58:1209–1217
Livesey E, Cortina Borja M, Sharif K et al (2009) Epidemiology of biliary atresia in England and Wales (1999–2006). Arch Dis Child Fetal Neonatal Ed 94:F451–F455
Schreiber RA, Barker CC, Roberts EA et al (2007) Biliary atresia: the Canadian experience. J Pediatr 151:659–665
Dong R, Shen Z, Zheng C, Chen G, Zheng S (2016) Serum microRNA microarray analysis identifies miR-4429 and miR-4689 are potential diagnostic biomarkers for biliary atresia. Sci Rep 6:21084
Zheng L, Lv Z, Gong Z, Sheng Q, Gao Z, Zhang Y, Yu S, Zhou J, Xi Z, Wang X (2017) Fn14 hepatic progenitor cells are associated with liver fibrosis in biliary atresia. Pediatr Surg Int 33:593–599
Makhmudi A, Kalim AS, Gunadi (2019) microRNA-21 expressions impact on liver fibrosis in biliary atresia patients. BMC Res Notes 12:189
Yu F, Zheng J, Mao Y, Dong P, Li G, Lu Z, Guo C, Liu Z (2015) Fan X (2015) Long non-coding RNA APTR promotes the activation of hepatic stellate cells and the progression of liver fibrosis. Biochem Biophys Res Commun 463:679–685
Tajima A, Pan IH, Fucharoen G, Fucharoen S, Matsuo M, Tokunaga K et al (2002) Three major lineages of Asian Y chromosomes: implications for the peopling of east and southeast Asia. Hum Genet 110:80–88
Gunadi, Makhmudi A, Agustriani N, Rochadi (2016) Effects of SEMA3 polymorphisms in Hirschsprung disease patients. Pediatr Surg Int 32:1025–1028
Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN (2012) The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 57:556–563
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Huang GC, Zhang JS, Tang QQ (2004) Involvement of C/EBP-alpha gene in in vitro activation of rat hepatic stellate cells. Biochem Biophys Res Commun 324:1309–1318
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J, Huang G et al (2012) Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients' poor recurrence-free survival after hepatectomy. Hepatology 56:2231–2241
Lorenzen JM, Schauerte C, Kielstein JT, Hübner A, Martino F, Fiedler J et al (2015) Circulating long noncoding RNATapSaki is a predictor of mortality in critically ill patients with acute kidney injury. Clin Chem 61:191–201
Yu S, Qi Y, Jiang J, Wang H, Zhou Q (2018) APTR is a prognostic marker in cirrhotic patients with portal hypertension during TIPS procedure. Gene 645:30–33
Mannan R, Misra V, Misra SP, Singh PA, Dwivedi M (2014) A comparative evaluation of scoring systems for assessing necro-inflammatory activity and fibrosis in liver biopsies of patients with chronic viral hepatitis. J Clin Diagn Res 8:FC08-12
Yang Z, Jiang S, Shang J, Jiang Y, Dai Y, Xu B et al (2019) LncRNA: shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev 52:17–31
Teng KY, Ghoshal K (2015) Role of noncoding RNAs as biomarker and therapeutic targets for liver fibrosis. Gene Expr 16:155–162
Gunadi, Budi NYP, Kalim AS, Santiko W, Musthofa FD, Iskandar K, Makhmudi A (2019) Aberrant expressions of miRNA-206 target, FN1, in multifactorial Hirschsprung disease. Orphanet J Rare Dis 14:5
Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y (2013) Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183:182–194
Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7:18
Cai X, Li J, Yuan X, Xiao J, Dooley S, Wan X, Weng H, Lu L (2018) CD133 expression in cancer cells predicts poor prognosis of non-mucin producing intrahepatic cholangiocarcinoma. J Transl Med 16:50
Acknowledgements
We want to give thanks to everybody who assisted the study. We are also thankful to an English editing service staff at the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada (FK-KMK UGM) for checking the manuscript grammar.
Funding
This study was funded by the FK-KMK UGM (Grant nos. UPPM/220/M/05/04/05.17 and UPPM/363/M/05/04/05.18 to G and AM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest.
Ethical approval
This study has an approval from the Ethical Committee of the FK-KMK UGM/Dr. Sardjito Hospital (KE/FK/1252/EC/2018 and KE/FK/1088/EC/2017).
Informed consent
Written informed consent was obtained from the parents’ BA patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makhmudi, A., Supanji, R., Putra, B.P. et al. The effect of APTR, Fn14 and CD133 expressions on liver fibrosis in biliary atresia patients. Pediatr Surg Int 36, 75–79 (2020). https://doi.org/10.1007/s00383-019-04582-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-019-04582-2