Abstract
Purpose
Several oxygen-dependent factors, e.g., CAIX (carbonic anhydrase IX) or phosphoglycerate kinase 1 (PGK1) interacting with the CXCR4/SDF1 axis (chemokine receptor 4/stromal cell derived factor 1) have been shown to be involved in processes of tumour pathology including tumourigenicity, tumour cell dissemination and poor survival in several solid tumour entities. The aim of the current study was to evaluate the influence of the hypoxia-inducible factors CAIX and PGK1 on progression of neuroblastoma and to evaluate the clinical relevance of possible therapeutic approaches.
Methods
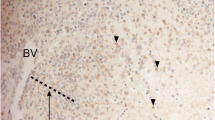
Expression of hypoxia-dependent factors PGK1 and CAIX was examined in neuroblastoma specimen, was correlated with clinical parameters, and was studied in neuroblastoma cells. The impact of these hypoxic factors was evaluated by proliferation assays under targeted therapy.
Results
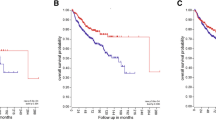
Expression of hypoxia-dependent factors was found in 50 % of neuroblastoma specimen. In neuroblastoma cells, CAIX and PGK1 expression is up regulated under hypoxia and correlates with response to targeted anti-proliferative treatment. The negative impact on survival, although significant for both CAIX and PGk1, appears to be stronger for CAIX.
Conclusions
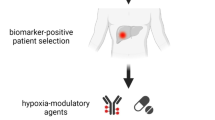
Our results show that the hypoxic factors in the tumour`s microenvironment further the progression of tumour disease. This strengthens the perspectives for additive novel therapeutic approaches targeting hypoxia-dependent factors in this childhood disease.



Similar content being viewed by others
References
Jubb AM, Buffa FM, Harris AL (2010) Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med 14:18–29
Kato Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J et al (2010) Expression of a hypoxia-associated protein, carbonic anhydrase-9, correlates with malignant phenotypes of gastric carcinoma. Digestion 82:246–251
Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, Supuran CT, Pastorekova S et al (2012) Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J Oral Pathol Med 41:667–674
Chen J, Rocken C, Hoffmann J, Kruger S, Lendeckel U et al (2005) Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut 54:920–927
Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C et al (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71:3364–3376
Fiaschi T, Giannoni E, Taddei ML, Cirri P, Marini A et al (2013) Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 12:1791–1801
Jung JH, Im S, Jung ES, Kang CS (2013) Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci 10:1217–1223
Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC et al (2013) Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32:5210–5219
Birner P, Jesch B, Friedrich J, Riegler M, Zacherl J et al (2011) Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol 18:3330–3337
Tanaka N, Kato H, Inose T, Kimura H, Faried A et al (2008) Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer 99:1468–1475
Ameis HM, Drenckhan A, von Loga K, Escherich G, Wenke K et al (2013) PGK1 as predictor of CXCR4 expression, bone marrow metastases and survival in neuroblastoma. PLoS ONE 8:e83701
Chaudary N, Hill RP (2007) Hypoxia and metastasis. Clin Cancer Res 13:1947–1949
Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F et al (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69:358–368
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia and cancer. J Mol Med (Berl) 85:1301–1307
Ameis HM, Drenckhan A, Freytag M, Izbicki JR, Supuran CT, et al. (2015) Carbonic anhydrase IX correlates with survival and is a potential therapeutic target for neuroblastoma. J Enzyme Inhib Med Chem 17:1–6
Brown JM, Wilson WR (2004) Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
Kimbro KS, Simons JW (2006) Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer 13:739–749
Boyle RG, Travers S (2006) Hypoxia: targeting the tumour. Anticancer Agents Med Chem 6:281–286
Fyles A, Milosevic M, Pintilie M, Syed A, Levin W et al (2006) Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother Oncol 80:132–137
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ (2006) Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 66:5216–5223
Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM (2002) MRI of the tumor microenvironment. J Magn Reson Imaging 16:430–450
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD (2009) The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem 284:20299–20310
Vaupel P (2004) The role of hypoxia-induced factors in tumor progression. Oncologist 9(Suppl 5):10–17
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 88: 1474–1480
Semenza GL (2006) Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91:803–806
Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S et al (2003) Investigating hypoxic tumor physiology through gene expression patterns. Oncogene 22:5907–5914
Lal A, Peters H, St Croix B, Haroon ZA, Dewhirst MW et al (2001) Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst 93:1337–1343
Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J et al (2006) Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J 25:4650–4662
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J et al (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60:7075–7083
Stolze IP, Mole DR, Ratcliffe PJ (2006) Regulation of HIF: prolyl hydroxylases. Novartis Found Symp 272:15–25 (discussion 25–36)
Semenza GL (2000) Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 35:71–103
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL et al (2008) Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem 283:20473–20483
Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR (2007) Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am J Physiol Cell Physiol 293:C738–C748
Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A et al (2011) Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 71:7558–7567
Potter C, Harris AL (2004) Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle 3:164–167
Acknowledgments
The authors thank Beate Roth and Brigit Appl for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. M. Ameis and A. Drenckhan contributed equally to this study.
Rights and permissions
About this article
Cite this article
Ameis, H.M., Drenckhan, A., Freytag, M. et al. Influence of hypoxia-dependent factors on the progression of neuroblastoma. Pediatr Surg Int 32, 187–192 (2016). https://doi.org/10.1007/s00383-015-3831-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3831-8