Abstract
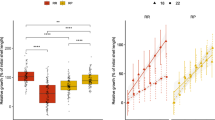
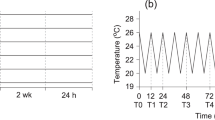
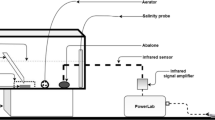
The underlying mechanisms controlling growth heterosis in marine invertebrates remain poorly understood. We used pure blacklip (Haliotis rubra) and greenlip (Haliotis laevigata) abalone, as well as their hybrid, to test whether differences in movement and/or aerobic versus anaerobic energy use are linked to a purported increased growth rate in hybrids. Abalone were acclimated to control (16 °C) and typical summer temperatures (23 °C), each with oxygen treatments of 100% air saturation (O2sat) or 70% O2sat. The experiment then consisted of two phases. During the first phase (chronic exposure), movement and oxygen consumption rates (ṀO2) of abalone were measured during a 2 day observation period at stable acclimation conditions. Additionaly, lactate dehydrogenase (LDH) and tauropine dehydrogenase (TDH) activities were measured. During phase two (acute exposure), O2sat was raised to 100% for abalone acclimated to 70% O2sat followed by an acute decrease in oxygen to anoxia for all acclimation groups during which movement and ṀO2 were determined again. During the chronic exposure, hybrids and H. laevigata moved shorter distances than H. rubra. Resting ṀO2, LDH and TDH activities, however, were similar between abalone types but were increased at 23 °C compared to 16 °C. During the acute exposure, the initial increase to 100% O2sat for individuals acclimated to 70% O2sat resulted in increased movement compared to individuals acclimated to 100% O2sat for hybrids and H. rubra when compared within type of abalone. Similarly, ṀO2 during spontaneous activity of all three types of abalone previously subjected to 70% O2sat increased above those at 100% O2sat. When oxygen levels had dropped below the critical oxygen level (Pcrit), movement in hybrids and H. laevigata increased up to 6.5-fold compared to movement above Pcrit. Differences in movement and energy use between hybrids and pure species were not marked enough to support the hypothesis that the purportedly higher growth in hybrids is due to an energetic advantage over pure species.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics Int 11:36–42
Allen VJ, Marsden ID, Ragg NL, Gieseg S (2006) The effects of tactile stimulants on feeding, growth, behaviour, and meat quality of cultured Blackfoot abalone, Haliotis iris. Aquaculture 257: 294–308
Alter K, Andrewartha SJ, Morash AJ, Clark TD, Hellicar AD, Leon RI, Elliott NG (2017) Hybrid abalone are more robust to multi-stressor environments than pure parental species. Aquaculture 478:25–34
Baldwin J, Wells RMG, Low M, Ryder JM (1992) Tauropine and D-lactate as metabolic stress indicators during transport and storage of live paua, (New Zealand abalone) (Haliotis iris). J Food Sci 57:280–282
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc Biol Sci 278:3465–3473
Buss JJ, Jones DA, Lumsden A, Harris JO, Bansemer MS, Stone DAJ (2015) Resticting feed ration has more effect than diet type on the feeding behaviour of greenlip abalone Haliotis laevigata. Mar Freshw Behav Phy 48:51–70
Calow P (1974) Some observations on locomotory strategies and their metabolic effects in two species of freshwater gastropods, Ancylus fluviatilis Müll. and Planorbis contortus Linn. Oecologia 16:149–161
Carefoot TH, Qian PY, Taylor BE, West T, Osborne J (1993) Effect of starvation on energy reserves and metabolism in the Northern abalone, Haliotis kamtschatkana. Aquaculture 118:315–325
Cenni F, Parisi G, Gherardi F (2009) Use of space and costs/benefits of locomotion strategies in the abalone, Haliotis tuberculata. Ethol Ecol Evol 21:15–26
Chacón O, Viana MT, Farías A, Vazquez C, García-Esquivel Z (2003) Circadian metabolic rate and short-term response of juvenile green abalone (Haliotis fulgens Philippi) to three anesthetics. J Shellfish Res 22(2):415–422
Cheng P, Liu X, Zhang G, Deng Y (2006) Heat-shock protein 70 gene expression in four hatchery Pacific abalone Haliotis discus hannai Ino populations using for marker-assisted selection. Aquac Res 37:1290–1296
Currie KL, Davidson H, Bansemer MS, Harris JO, Stone DA (2016) Ventral videographic assessment of the feeding behavior of juvenile greenlip [Haliotis laevigata (Donovan, 1808)] and hybrid (H. laevigata × Haliotis rubra) abalone in response to dietary and temperature manipulation. J Shellfish Res 35:641–651
Dahlhoff E, Somero GN (1993) Effects of temperature on mitochondria from abalone (Genus Haliotis) - adaptive plasticity and its limits. J Exp Biol 185:151–168
Denny M (1980) Locomotion: the cost of gastropod crawling. Science 208:1288–1290
Donovan D, Carefoot T (1997) Locomotion in the abalone Haliotis kamtschatkana: pedal morphology and cost of transport. J Exp Biol 200:1145–1153
Donovan DA, Carefoot TH (1998) Effect of activity on energy allocation in the northern abalone, Haliotis kamtschatkana (Jonas). J Shellfish Res 17:729–736
Donovan D, Baldwin J, Carefoot T (1999) The contribution of anaerobic energy to gastropod crawling and a re-estimation of minimum cost of transport in the abalone, Haliotis kamtschatkana (Jonas). J Exp Mar Biol Ecol 235:273–284
Elliott NG (2000) Genetic improvement programmes in abalone: what is the future? Aquac Res 31:51–59
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VI. Academic Press, London, pp 1–98
Gäde G (1988) Energy metabolism during anoxia and recovery in shell adductor and foot muscle of the gastropod mollusc Haliotis lamellosa: fomation of the novel anaerobic end product tauropine. Biol Bull 175:122–131
Gao X, Luo X, You W, Ke C (2020) Circadian movement behaviours and metabolism differences of the Pacific abalone Haliotis discus hannai. J Photochem Photobiol B Biol 211:111994
Gao X, Pang G, Luo X, You W, Ke C (2021) Effects of light cycle on motion behaviour and melatonin secretion in Haliotis discus hannai. Aquaculture 532: 735981.
Gaty G, Wilson JH (1986) Effect of body size, starvation, temperature, and oxygen tension on the oxygen consumption of hatchery-reared ormers Haliotis tuberculata L. Aquaculture 56:229–237
Gilroy A, Edwards SJ (1998) Optimum temperature for growth of Australian abalone: preferred temperature and critical thermal maximum for blacklip abalone, Haliotis rubra (Leach), and greenlip abalone, Haliotis laevigata (Leach). Aquac Res 29:481–485
Guo XM (2009) Use and exchange of genetic resources in molluscan aquaculture. Rev Aquac 1:251–259
Hamilton MG, Kube PD, Elliott NG, McPherson LJ, Krsinich A (2009) Development of a breeding strategy for hybrid abalone. Proc Assoc Advmt Anim Breed Genet 18:350–353
Harris JO, B Maguire GB, Edwards SJ, Johns DR (1999) Low dissolved oxygen reduces growth rate and oxygen consumption rate of juvenile greenlip abalone, Haliotis laevigata Donovan. Aquaculture 174:265-278
Hickey AJR, Wells RMG (2003) Thermal constraints on glycolytic metabolism in the New Zealand abalone, Haliotis iris: the role of tauropine dehydrogenase. New Zeal J Mar Fresh 37:723–731
Huntingford FA (2004) Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J Fish Biol 65:122–142
Jan RQ, Chang KH (1983) The oxygen consumption by Formosan abalone, Haliotis diÍersicolor supertexta Lishke, during decline of ambient oxygen. Bull Inst Zool Acad Sin 22:43–48
Kay M, Elkin L, Higgins J, Wobbrock JO (2021) ARTool: Aligned rank transform for nonparametric factorial ANOVAs. R package version 0.11.0. https://github.com/mjskay/ARTool
Kube PD, Appleyard SA, Elliott NG (2007) Selective breeding greenlip abalone (Haliotis laevigata): preliminary results and issues. J Shellfish Res 26:821–824
Lachambre S, Huchette S, Day R, Boudry P, Rio-Cabello A, Fustec T, Roussel S (2017) Relationships between growth, survival, physiology and behaviour—a multi-criteria approach to Haliotis tuberculata phenotypic traits. Aquaculture 467:190–197
Lee AC, Lee KT (2011) The enzyme activities of opine and lactate dehydrogenases in the gills, mantle, foot, and adductor of the hard clam Meretrix lusoria. J Mar Sci Technol 19:361–367
Leighton DL, Lewis AC (1982) Experimental hybridization in abalones. Int J Invertebr Reprod 5:273–282
Morash AJ, Alter K (2016) Effects of environmental and farm stress on abalone physiology: perspectives for abalone aquaculture in the face of global climate change. Rev Aquac 84:342–368
Muggeo VMR (2008) Segmented: an R package to fit regression models with broken-line relationships. R News 8:20–25
Myrick CA (2009) A low-cost system for capturing and analyzing the motion of aquatic organisms. J N Am Benthol Soc 28:101–109
Norin T, Malte H, Clark TD (2016) Differential plasticity of metabolic rate phenotypes in a tropical fish facing environmental change. Funct Ecol 30:369–378
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2021) nlme: linear and nonlinear mixed effects models. R package version 3.1–151. https://CRAN.R-project.org/package=nlme
Pörtner HO, Grieshaber MK (1993) Critical PO2 (s) in oxyconforming and oxyregulating animals: gas exchange, metabolic rate and the mode of energy production. In: Bicudo E (ed) The vertebrate gas transport cascade—adaptations to environment and mode of life. CRC Press, Boca Raton FL, pp 330-357
Pörtner HO, Dupont S, Melzner F, Storch D, Thorndyke M (2010) Studies of metabolic rate and other characters across life stages. Publications Office of the European Union, Luxembourg, pp 167-180
Prosser CL (1973) (ed) Comparative animal physiology. WB Saunders Company, Philadelphia, PA, USA, pp 966
Robinson N, Smith B, Cooke I, Strugnell J (2013) A snail’s pace: a preliminary analysis of the effects of stress and genetics on movement of Haliotis. Aquaculture 376:25–35
Schindelin J, Rueden CT, Hiner MC, Eliceiri KW (2015) The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82:518–529
Shepherd SA (1973) Studies on Southern Australian abalone (genus Haliotis). I. Ecology of five sympatric species. Mar Freshw Res 24:217–258
Tripp-Valdez MA, Bock C, Lannig G, Koschnick N, Pörtner HO, Lucassen M (2019) Assessment of muscular energy metabolism and heat shock response of the green abalone Haliotis fulgens (Gastropoda: Philipi) at extreme temperatures combined with acute hypoxia and hypercapnia. Comp Biochem Physiol B Biochem Mol Biol 227:1–11
Wassnig M, Day RW, Roberts RD, Krsinich A (2009) Effects of density and food ration on the growth rate, mortality and biomass return of abalone in slab tanks. Aquac Res 40:1501–1509
Wassnig M, Roberts RD, Krsinich A, Day RW (2010) Effects of water flow rate on growth rate, mortality and biomass return of abalone in slab tanks. Aquac Res 41:839–846
Wells RMG, Baldwin J, Speed SR, Weber RE (1998) Haemoxyanin function in the New Zealand abalones Haliotis iris and H. australis: relationships between oxygen-binding properties, muscle metabolism and habitat. Mar Freshwater Res 49:143–149
Acknowledgements
We thank Jade Tiger Abalone for providing abalone for this study. KA is grateful for support from the University of Tasmania, the Sense-T Program and the Commonwealth Scientific and Industrial Research Organisation for funding and scholarships. TDC is the recipient of an Australian Research Council Future Fellowship (FT180100154) funded by the Australian Government.
Funding
This research was funded by the University of Tasmania, the Sense-T Program and the Commonwealth Scientific and Industrial Research Organisation.
Author information
Authors and Affiliations
Contributions
KA, SJA, AJM, TDC, NGE and PBF developed the experimental design, KA conducted the experiments and SA analysed the data. KA wrote the manuscript and received constructive comments and revisions from all co-authors. All authors have approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by E. Polymeropoulos.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alter, K., Morash, A.J., Andrewartha, S.J. et al. Aerobic and anaerobic movement energetics of hybrid and pure parental abalone. J Comp Physiol B 191, 1111–1124 (2021). https://doi.org/10.1007/s00360-021-01388-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-021-01388-4