Abstract
Purpose
This study aimed to develop and validate an ultrasound (US)-based nomogram for the preoperative differentiation of renal urothelial carcinoma (rUC) from central renal cell carcinoma (c-RCC).
Methods
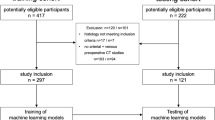
Clinical data and US images of 655 patients with 655 histologically confirmed malignant renal tumors (521 c-RCCs and 134 rUCs) were collected and divided into training (n = 455) and validation (n = 200) cohorts according to examination dates. Conventional US and contrast-enhanced US (CEUS) tumor features were analyzed to determine those that could discriminate rUC from c-RCC. Least absolute shrinkage and selection operator regression was applied to screen clinical and US features for the differentiation of rUC from c-RCC. Using multivariate logistic regression analysis, a diagnostic model of rUC was constructed and visualized as a nomogram. The diagnostic model’s performance was assessed in the training and validation cohorts by calculating the area under the receiver operating characteristic curve (AUC) and calibration plot. Decision curve analysis (DCA) was used to assess the clinical usefulness of the US-based nomogram.
Results
Seven features of both clinical features and ultrasound imaging were selected to build the diagnostic model. The nomogram achieved favorable discrimination in the training (AUC = 0.996, 95% CI: 0.993–0.999) and validation (AUC = 0.995, 95% CI: 0.974, 1.000) cohorts, and good calibration (Brier scores: 0.019 and 0.016, respectively). DCA demonstrated the clinical usefulness of the US-based nomogram.
Conclusion
A noninvasive clinical and US-based nomogram combining conventional US and CEUS features possesses good predictive value for differentiating rUC from c-RCC.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Roupret M, Babjuk M, Burger M et al (2021) European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol 79:62–79
Perez-Montiel D, Wakely PE, Hes O, Michal M, Suster S (2006) High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol 19:494–503
Ljungberg B, Albiges L, Abu-Ghanem Y et al (2022) European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 82:399–410
Motzer RJ, Jonasch E, Agarwal N et al (2022) Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20:71–90
Otto W, Shariat SF, Fritsche HM et al (2011) Concomitant carcinoma in situ as an independent prognostic parameter for recurrence and survival in upper tract urothelial carcinoma: a multicenter analysis of 772 patients. World J Urol 29:487–494
Lee CH, Ku JY, Jeong CW et al (2017) Predictors for intravesical recurrence following radical nephroureterectomy for upper tract urothelial carcinoma: a national multicenter analysis. Clin Genitourin Cancer 15:e1055–e1061
Soria F, Shariat SF, Lerner SP et al (2017) Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol 35:379–387
Veeratterapillay R, Geraghty R, Pandian R et al (2022) Ten-year survival outcomes after radical nephroureterectomy with a risk-stratified approach using prior diagnostic ureteroscopy: a single-institution observational retrospective cohort study. BJU Int 129:744–751
Guo RQ, Hong P, Xiong GY et al (2018) Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: a meta-analysis. BJU Int 121:184–193
Nowak L, Krajewski W, Chorbinska J et al (2021) The impact of diagnostic ureteroscopy prior to radical nephroureterectomy on oncological outcomes in patients with upper tract urothelial carcinoma: a comprehensive systematic review and meta-analysis. J Clin Med 10:4197
Mertens LS, Sharma V, Matin SF et al (2023) Bladder recurrence following upper tract surgery for urothelial carcinoma: a contemporary review of risk factors and management strategies. Eur Urol Open Sci 49:60–66
Kenigsberg AP, Meng X, Ghandour R, Margulis V (2020) Oncologic outcomes of radical nephroureterectomy (RNU). Transl Androl Urol 9:1841–1852
Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF (2012) Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol 61:1069–1070
Janisch F, Shariat SF, Baltzer P et al (2020) Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): a systematic review and meta-analysis. World J Urol 38:1165–1175
Rouviere O, Cornelis F, Brunelle S et al (2020) Imaging protocols for renal multiparametric MRI and MR urography: results of a consensus conference from the French Society of Genitourinary Imaging. Eur Radiol 30:2103–2114
Taylor JI, Souter LH, Barocas DA, Boorjian SA, Raman JD, Lotan Y (2023) Diagnostic imaging in the evaluation of asymptomatic microhematuria: systematic review and meta-analysis. J Urol 209:1099–1106
Benzon HT, Maus TP, Kang HR et al (2021) The use of contrast agents in interventional pain procedures: a multispecialty and multisociety practice advisory on nephrogenic systemic fibrosis, gadolinium deposition in the brain, encephalopathy after unintentional intrathecal gadolinium injection, and hypersensitivity reactions. Anesth Analg 133:535–552
Olson MC, Abel EJ, Mankowski GL (2019) Contrast-enhanced ultrasound in renal imaging and intervention. Curr Urol Rep 20:73
Furrer MA, Spycher S, Buttiker SM et al (2020) Comparison of the diagnostic performance of contrast-enhanced ultrasound with that of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging in the evaluation of renal masses: a systematic review and meta-analysis. Eur Urol Oncol 3:464–473
Zhang B, Li J, Wu Z et al (2021) Contrast-enhanced ultrasound characteristics of renal pelvis urothelial carcinoma and its relationship with microvessel density. Ultrasound Med Biol 47:236–243
Xue LY, Lu Q, Huang BJ et al (2013) Evaluation of renal urothelial carcinoma by contrast-enhanced ultrasonography. Eur J Radiol 82:e151–e157
Wehrli NE, Kim MJ, Matza BW, Melamed J, Taneja SS, Rosenkrantz AB (2013) Utility of MRI features in differentiation of central renal cell carcinoma and renal pelvic urothelial carcinoma. Am J Roentgenol 201:1260–1267
Wang T, Gao T, Guo H et al (2020) Preoperative prediction of parametrial invasion in early-stage cervical cancer with MRI-based radiomics nomogram. Eur Radiol 30:3585–3593
Aheto J, Duah HO, Agbadi P, Nakua EK (2021) A predictive model, and predictors of under-five child malaria prevalence in Ghana: how do LASSO, ridge and elastic net regression approaches compare? Prev Med Rep 23:101475
Rai BP, Luis DEJ, Vale L et al (2022) Systematic review of the incidence of and risk factors for urothelial cancers and renal cell carcinoma among patients with haematuria. Eur Urol 82:182–192
Musaddaq B, Musaddaq T, Gupta A, Ilyas S, von Stempel C (2020) Renal cell carcinoma: the evolving role of imaging in the 21st century. Semin Ultrasound CT MRI 41:344–350
Schieda N, Lim RS, McInnes M et al (2018) Characterization of small (<4cm) solid renal masses by computed tomography and magnetic resonance imaging: current evidence and further development. Diagn Interv Imaging 99:443–455
Tsili AC, Andriotis E, Gkeli MG et al (2021) The role of imaging in the management of renal masses. Eur J Radiol 141:109777
Danzig MR, Mallin K, McKiernan JM et al (2018) Prognostic importance of lymphovascular invasion in urothelial carcinoma of the renal pelvis. Cancer 124:2507–2514
Funding
Natural Science Foundation (CN), 12174074, Bei-jian Huang, Shanghai Municipal Health Commission, R2021-007, Cuixian Li, 202240153, Cuixian Li.
Author information
Authors and Affiliations
Contributions
LC: protocol/project development, data collection and management, and manuscript writing; LB: protocol/project development, data collection and management; ZQ: data analysis, manuscript writing/editing; LQ: manuscript editing; WJ: data collection and management; SP: and data collection and management; XH: conceptualization and supervision; HB: conceptualization, data collection, validation, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest.
Ethical approval
The studies involving human participants were reviewed and approved by the institutional Ethics Review Board (Approval No.: B2021-290R, Approval Date: 2021.5.19).
Informed consent
Written consent from the patients was waived for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Lu, B., Zhao, Q. et al. Development and validation of a clinical and ultrasound features-based nomogram for preoperative differentiation of renal urothelial carcinoma and central renal cell carcinoma. World J Urol 42, 227 (2024). https://doi.org/10.1007/s00345-024-04935-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04935-0