Abstract
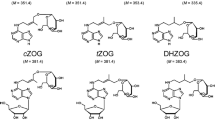
Qualitative and quantitative analyses were carried out on vegetative tissues of potato (Solanum tuberosum cv. “Katahdin”) in search of natural products thought to play a role in tuber induction. Tissues were obtained from plants initially grown in a growth chamber under noninducing conditions (30°C day and 28°C night with an 18-h photoperiod), and then half of the plants were moved to inducing chambers (28°C day and 13°C night with a 10-h photoperiod) for 10 days prior to tissue harvest. Plants from each chamber were then harvested at 2-day intervals for 10 days, separated into above- and belowground portions, and the lyophilized tissues were extracted and subjected to rigorous purification and separation using high-performance liquid chromatography. This was followed by identification and quantification using combined gas chromatography-mass spectrometry. Compounds isolated and identified included gibberellic acid; cytokinins cis-zeatin riboside, trans-zeatin, trans-zeatin riboside, and isopentenyladenine; and jasmonates jasmonic acid, tuberonic acid and its methyl ester, methyl 7-isocucurbate, and 9,10-dihydromethyljasmonate. Methyl 7-isocucurbate and 9,10-dihydromethyljasmonate were detected for the first time in potato tissue as endogenous compounds. Cytokinin and jasmonate levels generally increased under inducing conditions, whereas gibberellic acid levels declined progressively during the 10-day sampling period. Only gibberellic acid, jasmonic acid, and cis-zeatin riboside levels were significantly influenced by induction.



Similar content being viewed by others
References
Abdala G, Castro G, Guinazu MM., Tizio R, Miersch O (1996) Occurrence of jasmonic acid in organs of Solanum tuberosum L. and its effect on tuberization. Plant Growth Regul 19:139–143
Auer CA (1997) Cytokinin conjugation: recent advances and patterns in plant evolution. Plant Growth Regul 23:17–32
Bassil NV, Mok DWS, Mok MC (1993) Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiol 102:867–872
Cenzano A, Vigliocco A, Kraus T, Abdala G (2003) Exogenously applied jasmonic acid induces changes in apical meristem morphology of potato stolons. Ann Bot 91:915–919
Chapman HW (1958) Tuberization in the potato plant. Physiol Plant 11:215–224
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48:355–381
Dam JV, Kooman PL, Struik PC (1996) Effect of temperature and photoperiod on early growth and final number of tubers in potato (Solanum tuberosum L.). Potato Res 39:51–62
Dermastia M, Ravnikar M, Vilhar B, Kovac M (1994) Increased level of cytokinin ribosides in jasmonic acid treated potato (Solanum tuberosum) stem node cultures. Physiol Plant 92:241–246
Ewing EE (1995) The role of hormones in potato (Solanum tuberosum L.) tuberization. In: Davies PJ (ed), Plant Hormones: Physiology, Biochemistry and Molecular Biology, 2nd edn. Dordrecht, The Netherlands: Kluwer Academic Publishers, pp 698–724
Ewing EE, Wareing PF (1978) Shoot, stolon, and tuber formation on potato (Solanum tuberosum L.) cuttings in response to photoperiod. Plant Physiol 61:348–353
Fernie AR, Willmitzer L (2001) Molecular and biochemical triggers of potato tuber development. Plant Physiol 127:1459–1465
Forsline PL, Langille AR (1975) Endogenous cytokinins in Solanum tuberosum as influenced by photoperiod and temperature. Physiol Plant 34:75–77
Fujino K, Koda Y, Kikuta K (1995) Reorientation of cortical microtubules in the sub-apical region during tuberization in single node stem segments of potato in culture. Plant Cell Physiol 36:891–895
Gemal AL, Luche JL (1981) The reduction of α-enones by sodium borohydride in the presence of lanthanoid chlorides: synthetic and mechanistic aspects. J Am Chem Soc 103:5454–5459
Glenn JL, Kuo CC, Durley RC, Pharis RP (1972) Use of insoluble polyvinylpyrrolidone for purification of plant extracts and chromatography of plant hormones. Phytochemistry 11:345–351
Gregory LE (1956) Some factors for tuberization in the potato plant. Am J Bot 43: 281–288
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Helder H, Miersch O, Vreugdenhil D, Sembdner G (1993) Occurrence of hydroxylated jasmonic acids in leaflets of Solanum demissum plants grown under long- and short-day conditions. Physiol Plant 88:647–653
Ivana M, Lidiya S, Milos O, Oksana Z, Tatyana K, Josef E, Jaroslava O, Svetlana G, Yurii R, Nina A (1997) Growth pattern, tuber formation and hormonal balance in in vitro potato plants carrying ipt gene. Plant Growth Regul 21:27–36
Jackson SD (1999) Mutiple signaling pathways control tuber induction in potato. Plant Physiol 119:1–8
Kitahara T, Iwamoto M, Takagi Y, Mori K, Matsui M (1984) The synthesis of jasmine ketolactone. Agric Biol Chem 48:1731–1734
Kiyota H, Saitoh M, Oritani T, Yoshihara T (1996) Synthesis and potato tuber inducing activity of methyl 5′, 5′, 5′-trifluorojasmonate. Phytochemistry 42:1259–1262
Klebe JF, Finkbeiner H, White DM (1966) Silylation with bis(trimethylsilyl)acetamide, a highly reactive donor. J Am Chem Soc 88:3390–3395
Koda Y (1992) The role of jasmonic acid and related compounds in the regulation of plant development. Int Rev Cytol 135:155–199
Koda Y (1997) Possible involvement of jasmonates in various morphogenic events. Physiol Plant 100:639–646
Koda Y, Okazawa Y (1988) Detection of potato tuber-inducing activity in potato leaves and old tubers. Plant Cell Physiol 29:969–974
Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T (1991) Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry 30:1435–1438
Koda Y, Kikuta Y, Kitahara T, Nishi T, Mori K (1992) Comparison of various biological activities of stereoisomers of methyl jasmonate. Phytochemistry 31:1111–1114
Kolomiets MV, Hannapel DJ, Chen H, Tymeson M, Gladon RJ (2001) Lipoxygenase is involved in the control of potato tuber development. Plant Cell 13:613–626
Kramell R, Schmidt J, Schneider G, Sembdner G, Schreiber K (1988) Synthesis of N-(jasmonoyl)amino acid conjugates. Tetrahedron 44:5791–5807
Krauss A, Marschner H (1982) Influence of nitrogen nutrition, day length and temperature on contents of gibberellic and abscisic acid on tuberization of potato plants. Potato Res 25:13–21
Kumar D, Wareing PF (1974) Studies on the tuberization of Solanum andigena. II. Growth, hormones and tuberization. New Phytol 73:833–840
Kuroha T, Kato H, Asami T, Yoshida S, Kamada H, Satoh S (2002) A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J Exp Bot 53:2193–2200
Langille AR, Forsline PL (1974) Influence of temperature and photoperiod on cytokinin pools in the potato Solanum tuberosum L. Plant Sci Lett 2:189–191
Langille AR, Hepler PR (1992) Effect of three anti-gibberellin growth retardants on tuberization of induced and non-induced katahdin potato leaf-bud cuttings. Am Potato J 69:131–141
Letham DS, Palni LMS (1983) The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol 34:163–197
Martinez-Garcia JF, Garcia-Martinez JL, Bou J, Prat S (2002) The interaction of gibberellins and photoperiod in the control of potato tuberization. J Plant Growth Regul 20:377–386
Matsuki T, Tazaki H, Fujimore T, Hogetsu T (1990) The influence of jasmonic acid methyl ester on microtubules in potato cells and formation of potato tubers. Biosci Biotech Biochem 56:1329–1330
Mauk CS, Langille AR (1978) Physiology of tuberization in Solanum tuberosum L. cis-zeatin riboside in the potato plant: its identification and changes in endogenous levels as influenced by temperature and photoperiod. Plant Physiol 62:438–442
Miersch O, Preiss A, Sembdner G, Schreiber K (1987) (+)-iso-Jasmonic acid and related compounds from botrydiplodia theobromae. Phytochemistry 26:1037–1039
Melis RJM, van Staden J (1984) Tuberization and hormones. Z Pflanzenphysiol 113:271–283
Menzel CM (1983) Tuberization in potato at high temperatures: gibberellin content and transport from buds. Ann Bot 52:697–702
Nicander B, Bjorkman PO, Tillberg E (1995) Identification of an N-glucoside of cis-zeatin from potato tuber sprouts. Plant Physiol 109:513–516
Nojiri H, Yamane H, Seto H, Yamaguchi I, Murofushi N, Yoshihara T, Shibaoka H (1992) Qualitative and quantitative analysis of endogenous jasmonic acid in bulbing and non-bulbing onion plants. Plant Cell Physiol 33:1225–1231
Palmer CE, Smith OE (1969) Cytokinins and tuber initiation in the potato Solanum tuberosum L. Nature 221:279–280
Pelacho AM, Mingo-Castel AM (1991) Jasmonic acid induces tuberization of potato stolons cultured in vitro. Plant Physiol 97:1253–1255
Railton ID, Wareing PE (1973) Effects of daylength on endogenous gibberellins in leaves of solanum andigena I. Changes in levels of free acidic gibberellin-like substances. Physiol Plant 28:88–94
Sembdner G, Parthier B (1993) The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol 44:569–589
Schaller F, Schaller A, Stintzi A (2005) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23:179–199
Shibaoka H (1993) Regulation by gibberellins of the orientation of cortical microtubules in plant cells. Aust J Plant Physiol 20:461–470
Shibaoka H (1994) Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 45:527–544
Slater JW (1968) The effect of night temperature on tuber initiation of the potato. Eur Potato J 11:14–22
Thenot JP, Horning EC, Stafford M, Horning MG (1972) Fatty acids esterification with N,N-dimethylformamide dialkyl acetals for GC analysis. Anal Lett 5:217–223
Trewavas AJ (1983) Is plant development regulated by changes in the concentration of plant growth substances? Trends Biochem Sci 8:354–356
Van den Berg JH, Ewing EE (1991) Jasmonates and their role in plant growth and development with special reference to the control of potato tuberization: a review. Am Potato J 68:781–794
Veach YK, Martin RC, Mok DWS, Malbeck J, Vankova R, Mok MC (2003) O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131:1374–1380
Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol 75:458–461
Vreugdenhil D, Struik PC (1989) An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiol Plant 75:525–531
Xu X, van Lammeren AA, Vermeer E, Vreugdenhil D (1998) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Yoshihara T, Amanuma M, Tsutsumi T, Okumura Y, Matsuura H, Ichihara A (1996) Metabolism and transport of [2-14C]-(+/−)-jasmonic acid in the potato plant. Plant Cell Physiol 37:586–590
Yoshihara T, Omer EA, Koshino H, Sakamura S, Kikuta Y, Koda Y (1989) Structure of a tuber-inducing stimulus from potato leaves (Solanum tuberosum L.). Agric Biol Chem 53:2835–2837
Woolley DJ, Wareing PF (1972) Environmental effects on endogenous cytokinins and gibberellin levels in Solanum tuberosum. New Phytol 71:1015–1025
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malkawi, A., Jensen, B.L. & Langille, A.R. Plant Hormones Isolated from “Katahdin” Potato Plant Tissues and the Influence of Photoperiod and Temperature on Their Levels in Relation to Tuber Induction. J Plant Growth Regul 26, 308–317 (2007). https://doi.org/10.1007/s00344-007-9010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-007-9010-y