Abstract
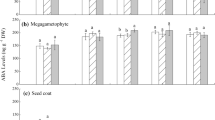
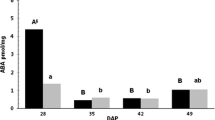
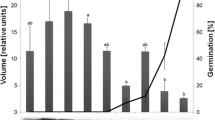
To investigate the role of abscisic acid (ABA) biosynthesis and catabolism in dormant imbibed seeds of western white pine (Pinus monticola), ABA and selected catabolites were measured during a combined treatment of the ABA biosynthesis inhibitor fluridone, and gibberellic acid (GA). Fluridone in combination with GA effectively disrupted ABA homeostasis and replaced the approximately 90-day moist chilling period normally required to break dormancy in this species. Individually, both fluridone and GA treatments decreased ABA levels in the embryos and megagametophytes of white pine seeds compared to a water control; however, combined fluridone/GA treatment, the only treatment to terminate dormancy effectively, led to the greatest decline in ABA content. Fluridone treatments revealed that a high degree of ABA turnover/transport occurred in western white pine seeds during the initial stages of dormancy maintenance; at this time, ABA levels decreased by approximately two-thirds in both embryo and megagametophyte tissues. Gibberellic acid treatments, both alone and in combination with fluridone, suggested that GA acted transiently to disrupt ABA homeostasis by shifting the ratio between biosynthesis and catabolism to favor ABA catabolism or transport. Increases in phaseic acid (PA) and dihydrophaseic acid (DPA) were observed during fluridone/GA treatments; however, increases in ABA metabolites did not account for the reduction in ABA observed; additional catabolism and/or transport of ABA and selected metabolites in all probability accounts for this discrepancy. Finally, levels of 7′ hydroxy-ABA (7′OH-ABA) were higher in dormant-imbibed seeds, suggesting that metabolism through this pathway is increased in seeds that maintain higher levels of ABA, perhaps as a means to further regulate ABA homeostasis.




Similar content being viewed by others
References
Ali-Rachedi S, Bouinot D, Wagner M-H, Bonnet M, Sotta B, et al. 2004. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219:479–488
Bartels PG, Watson CW. 1978. Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci 26:198–203
Barthe P, Hogge LR, Abrams SR, Le Page-Degivry MT. 1993. Metabolism of (+) abscisic acid to dihydrophaseic acid-4′-β-D-glucopyranoside by sunflower embryos. Phytochemistry 34:645–648
Bewley JD, Black M (eds.). 1994. Seeds: Physiology of Development and Germination, 2nd ed. New York, NY, USA: Plenum Publishing Corp. p 445
Bianco J, Garello G, Le Page-Degivry MT. 1997. De novo ABA synthesis and expression of seed dormancy in a gymnosperm: Pseudotsuga menziesii. Plant Growth Regul 21:115–119
Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, et al. 2005. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin, and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42:35–48
Corbineau F, Bianco J, Garello G, Côme D. 2002. Breakage of Pseudotsuga menziesii seed dormancy by cold treatment as related to changes in seed ABA sensitivity and ABA levels. Physiol Plant 114:313–319
Dumroese RK. 2000. Germination-enhancing techniques for Pinus monticola seeds and speculation on seed dormancy. Seed Sci Technol 28:201–209
Feurtado JA, Xia J-H, Ma Y, Kermode AR. 2003. Increasing the temperature of the water soak preceding moist chilling promotes dormancy-termination in seeds of western white pine (Pinus monticola Dougl.). Seed Sci Technol 31:275–288
Feurtado JA, Ambrose SJ, Cutler AJ, Ross ARS, Abrams SR, Kermode AR. 2004. Dormancy termination of western white pine (Pinus monticola Dougl. Ex D. Don) seeds is associated with changes in abscisic acid metabolism. Planta 218:630–639
Finkelstein RR, Rock CD. 2002. Abscisic acid biosynthesis and response. In: Somerville CR, Meyerowitz EM (eds). The Arabidopsis Book, American Society of Plant Biologists, Rockville, MD, USA. doi/10.1199/tab.0058, http://www.aspb.org/publications/arabidopsis/
Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, et al. 2004. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot 55:111–118
Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M. 2000. Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210:279–285
Hoff RJ. 1987. Dormancy in Pinus monticola seed related to stratification time, seed coat, and genetics. Can J Forest Res 17:294–298
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, et al. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23:1647–1656
Le Page-Degivry MT, Garello G. 1992. In situ abscisic acid synthesis. A requirement for induction of embryo dormancy in Helianthus annus. Plant Physiol 98:1386–1390
Le Page-Degivry MT, Garello G, Barthe P. 1997. Changes in abscisic acid biosynthesis and catabolism during dormancy breaking in Fagus sylvatica embryo. J Plant Growth Regul 16:57–61
Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, et al. 2006. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J 45: 942–954
Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, et al. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134:1439–1449
Schmitz N, Xia J-H, Kermode AR. 2001. Dormancy of yellow-cedar seeds is terminated by gibberellic acid in combination with fluridone or with osmotic priming and moist chilling. Seed Sci Technol 29:331–346
Setter TL, Brenner ML, Brun WA, Krick TP. 1981. Identification of a dihydrophaseic acid aldopyranoside from soybean tissue. Plant Physiol 68:93–95
Wang M, Heimovaara-Dijkstra S, Van Duijn B. 1995. Modulation of germination of embryos isolated from dormant and non-dormant barley grains by manipulation of endogenous abscisic acid. Planta 195:586–592
Yoshioka T, Endo T, Satoh S. 1998. Restoration of seed germination at supraoptimal temperature by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39: 307–312
Zeevaart JAD. 1999. Abscisic acid metabolism and its regulation. In Hooykaas PJJ, Hall KR, Libbenga KR (eds), Biochemistry and Molecular Biology of Plant Hormones. Elsevier Science BV, Amsterdam, The Netherlands, pp. 189–207
Zhou R, Cutler AJ, Ambrose SJ, Galka MM, Nelson KM, et al. 2004. A new abscisic acid catabolic pathway. Plant Physiol 134:361–369
Acknowledgments
The authors thank Dave Kolotelo and Dean Christianson of the British Columbia Ministry of Forests, BC, Canada, for helping obtain mature seed of western white pine. The authors also thank Dr. Andrew R. S. Ross for suggested improvements to the manuscript.
This research was supported by Natural Sciences and Engineering Research Council of Canada (Strategic) and Protein Engineering Network of Centres of Excellence (PENCE) grants awarded to A.R.K., S.R.A., A.J.C., A.R.S.R., and other investigators.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feurtado, J.A., Yang, J., Ambrose, S.J. et al. Disrupting Abscisic Acid Homeostasis in Western White Pine (Pinus monticola Dougl. Ex D. Don) Seeds Induces Dormancy Termination and Changes in Abscisic Acid Catabolites. J Plant Growth Regul 26, 46–54 (2007). https://doi.org/10.1007/s00344-006-0035-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-006-0035-4