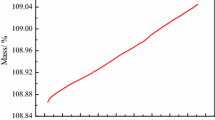
Abstract
In this study, isothermal oxidation behavior of a Cu–Al–Ni–Fe shape-memory alloy between 500 and 900 °C was investigated. Alloy samples were exposed to oxygen by TG/DTA for 1 h at a constant temperature, allowing for calculation of the oxidation constant and activation energy values of the oxidation process. The oxidation constant value increased with temperature, reaching saturation at 800 °C. The effect of oxidation on crystal structure, surface morphology and chemical composition of the Cu–Al–Ni–Fe alloy was determined by X-ray diffractometer (XRD) and scanning electron microscope (SEM)–energy-dispersive X-ray (EDX) analyses. With increasing oxidation temperature, number and intensity of the characteristic 18R martensite phase peaks were reduced while Al2O3 phase peaks were increased. In parallel to the XRD results, the same variations were also detected by SEM–EDX measurements.





Similar content being viewed by others
References
K. Otsuka, X. Ren, Recent developments in the research of shape memory alloys. Intermetallics 7, 511–528 (1999)
R. Zengin, Microstructure and oxidation properties of a neutron-irradiated Cu-13.5 wt% Al-4 wt% Ni shape memory alloy. Phys. B 363, 110–114 (2005)
A. Planes, L. Mañosa, D. Ríos-Jara, J. Ortín, Martensitic transformation of Cu-based shape-memory alloys: elastic anisotropy and entropy change. Phys. Rev. B 45, 7633–7639 (1992)
Y. Sutou, T. Omori, K. Yamauchi, N. Ono, R. Kainuma, K. Ishida, Effect of grain size and texture on pseudoelasticity in Cu–Al–Mn-based shape memory wire. Acta. Mater. 53, 4121–4133 (2005)
G. Lojen, M. Gojić, I. Anžel, Continuously cast Cu–Al–Ni shape memory alloy—properties in as-cast condition. J. Alloy. Compd. 580, 497–505 (2013)
J.I. Pérez-Landazábal, V. Recarte, V. Sánchez-Alarcos, M.L. Nó, J.S. Juan, Study of the stability and decomposition process of the β phase in Cu–Al–Ni shape memory alloys. Mater. Sci. Eng. A 438, 734–737 (2006)
P.P. Rodríguez, A. Ibarra, A. Iza-Mendia, V. Recarte, J.I. Pérez-Landazábal, J.S. Juan, M.L. Nó, Influence of thermo-mechanical processing on the microstructure of Cu-based shape memory alloys produced by powder metallurgy. Mater. Sci. Eng. A 378, 263–268 (2004)
G.R. Wallwork, The oxidation of alloys. Rep. Prog. Phys. 39, 401–485 (1976)
G.C. Wood, F.H. Stott, Oxidation of alloys. Mater. Sci. Technol. 3, 519–529 (1987)
Z. Cao, Y. Shen, F. Li, L. Yu, Oxidation of a quaternary two-phase Cu-40 Ni-17.5 Cr-2.5 Al alloy at 973–1073 K in 101 kPa O2. J. Alloy. Compd. 480, 449–453 (2009)
B. Yujun, L. Chengwei, G. Guili, Y. Longwei, TEM observation of oxidation of CuZnAlMnNi shape memory alloy. Chin. Sci. Bull. 46, 1837–1839 (2001)
Y.-J. Bai, Y.-X. Liu, D.-S. Sun, X.-F. Bian, L.-M. Xiao, G.-L. Geng, Atmospheric oxidation of CuZnAlMnNi shape memory alloy. Mater. Lett. 46, 358–361 (2000)
B.I. Portillo, S.K. Varma, Oxidation behavior of Nb-20Mo-15Si-5B-20Ti alloy in air from 700 to 1300 °C. J Alloy. Compd. 497, 68–73 (2010)
C.H. Xu, X.Q. Ma, S.Q. Shi, C.H. Woo, Oxidation behavior of TiNi shape memory alloy at 450–750 °C. Mater. Sci. Eng. A 371, 45–50 (2004)
M. Kök, G. Pirge, Y. Aydogdu, Isothermal oxidation study on NiMnGa ferromagnetic shape memory alloy at 600–1000 °C. Appl. Surf. Sci. 268, 136–140 (2013)
C. Tatar, R. Zengin, The effects of neutron irradiation on oxidation behavior, microstructure and transformation temperatures of Cu-12.7 wt% Al-5 wt% Ni-2 wt% Mn shape memory alloy. Thermochim. Acta 433, 56–58 (2005)
C. Tatar, R. Zengin, The effects of neutron irradiation on shape memory properties and oxidation behaviour of a Cu-13Al-4Ni alloy. Int. J. Therm. Sci. 47, 899–902 (2008)
R. Zengin, S. Ozgen, M. Ceylan, Oxidation behaviour and kinetics properties of shape memory CuAl x Ni4 (x = 13.0 and 13.5) alloys. Thermochim. Acta 414, 79–84 (2004)
R.G. Reddy, X. Wen, M. Divakar, Isothermal oxidation of TiAl alloy. Metall. Mater. Trans. A 32, 2357–2361 (2001)
F. Dagdelen, E. Ercan, The surface oxidation behavior of Ni-45.16% Ti shape memory alloys at different temperatures. J. Therm. Anal. Calorim. 115, 561–565 (2014)
K. Yildiz, M. Kök, Study of martensite transformation and microstructural evolution of Cu–Al–Ni–Fe shape memory alloys. J. Therm. Anal. Calorim. 115, 1509–1514 (2014)
Acknowledgments
We acknowledge Dr. Selçuk Aktürk (Materials Research Lab., Mugla Sitki Kocman University) for the SEM–EDX observations and analyses. We also wish to thank Professor Yusuf Atıcı (Firat University) and Professor Yıldırım Aydoğdu (Gazi University) due to their helpful supporting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kök, M., Yildiz, K. Oxidation parameters determination of Cu–Al–Ni–Fe shape-memory alloy at high temperatures. Appl. Phys. A 116, 2045–2050 (2014). https://doi.org/10.1007/s00339-014-8394-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8394-3