Abstract
Objectives
This study aimed to externally validate the Birmingham Atypical Cartilage Tumour Imaging Protocol (BACTIP) recommendations for differentiation/follow-up of central cartilage tumours (CCTs) of the proximal humerus, distal femur, and proximal tibia and to propose BACTIP adaptations if the results provide new insights.
Methods
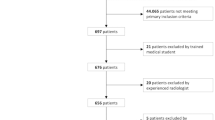
MRIs of 123 patients (45 ± 11 years, 37 men) with an untreated CCT with MRI follow-up (n = 62) or histopathological confirmation (n = 61) were retrospectively/consecutively included and categorised following the BACTIP (2003–2020 / Ghent University Hospital/Belgium). Tumour length and endosteal scalloping differences between enchondroma, atypical cartilaginous tumour (ACT), and high-grade chondrosarcoma (CS II/III/dedifferentiated) were evaluated. ROC-curve analysis for differentiating benign from malignant CCTs and for evaluating the BACTIP was performed.
Results
For lesion length and endosteal scalloping, ROC-AUCs were poor and fair-excellent, respectively, for differentiating different CCT groups (0.59–0.69 versus 0.73–0.91). The diagnostic performance of endosteal scalloping and the BACTIP was higher than that of lesion length. A 1° endosteal scalloping cut-off differentiated enchondroma from ACT + high-grade chondrosarcoma with a sensitivity of 90%, reducing the potential diagnostic delay. However, the specificity was 29%, inducing overmedicalisation (excessive follow-up). ROC-AUC of the BACTIP was poor for differentiating enchondroma from ACT (ROC-AUC = 0.69; 95%CI = 0.51–0.87; p = 0.041) and fair-good for differentiation between other CCT groups (ROC-AUC = 0.72–0.81). BACTIP recommendations were incorrect/unsafe in five ACTs and one CSII, potentially inducing diagnostic delay. Eleven enchondromas received unnecessary referrals/follow-up.
Conclusion
Although promising as a useful tool for management/follow-up of CCTs of the proximal humerus, distal femur, and proximal tibia, five ACTs and one chondrosarcoma grade II were discharged, potentially inducing diagnostic delay, which could be reduced by adapting BACTIP cut-off values.
Clinical relevance statement
Mostly, Birmingham Atypical Cartilage Tumour Imaging Protocol (BACTIP) assesses central cartilage tumours of the proximal humerus and the knee correctly. Both when using the BACTIP and when adapting cut-offs, caution should be taken for the trade-off between underdiagnosis/potential diagnostic delay in chondrosarcomas and overmedicalisation in enchondromas.
Key Points
• This retrospective external validation confirms the Birmingham Atypical Cartilage Tumour Imaging Protocol as a useful tool for initial assessment and follow-up recommendation of central cartilage tumours in the proximal humerus and around the knee in the majority of cases.
• Using only the Birmingham Atypical Cartilage Tumour Imaging Protocol, both atypical cartilaginous tumours and high-grade chondrosarcomas (grade II, grade III, and dedifferentiated chondrosarcomas) can be misdiagnosed, excluding them from specialist referral and further follow-up, thus creating a potential risk of delayed diagnosis and worse prognosis.
• Adapted cut-offs to maximise detection of atypical cartilaginous tumours and high-grade chondrosarcomas, minimise underdiagnosis and reduce potential diagnostic delay in malignant tumours but increase unnecessary referral and follow-up of benign tumours.
Graphical Abstract








Similar content being viewed by others
Abbreviations
- °:
-
Degree
- 2D/3D:
-
Two-/three-dimensional
- ACT:
-
Atypical cartilaginous tumour
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area-under-the-curve
- BACTIP:
-
Birmingham Atypical Cartilage Tumour Imaging Protocol
- CCT:
-
Central cartilage tumour
- cm:
-
Centimetre
- CS:
-
Chondrosarcoma
- DCE:
-
Dynamic contrast-enhanced
- DDCS:
-
Dedifferentiated chondrosarcoma
- DWI:
-
Diffusion-weighted imaging
- ETL:
-
Echo train length
- FOV:
-
Field-of-view
- HP:
-
Histopathological
- ICC:
-
Intraclass correlation coefficient
- IQR:
-
Interquartile range
- IVIM:
-
Intravoxel incoherent motion
- Kep:
-
Rate constant
- Ktrans:
-
Volume transfer constant
- mm:
-
Millimetre
- ms:
-
Millisecond
- n:
-
Number
- NA:
-
Not available
- NPV:
-
Negative predictive value
- PD:
-
Proton density
- PPV:
-
Positive predictive value
- Q:
-
Quartile
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- SUVmax:
-
Maximum standardised uptake value
- T:
-
Tesla
- T1:
-
T1-weighted
- T2:
-
T2-weighted
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
References
Deckers C, de Rooy JWJ, Flucke U et al (2021) Midterm MRI follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. Cancers (Basel). https://doi.org/10.3390/cancers13164093
Deng XY, Chen HY, Yu JN et al (2021) Diagnostic value of CT- and MRI-based texture analysis and imaging findings for grading cartilaginous tumors in long bones. Front Oncol. https://doi.org/10.3389/fonc.2021.700204
Patel A, Davies AM, Botchu R et al (2019) A pragmatic approach to the imaging and follow-up of solitary central cartilage tumours of the proximal humerus and knee. Clin Radiol. https://doi.org/10.1016/j.crad.2019.01.025
Sharif B, Lindsay D, Saifuddin A (2021) The role of imaging in differentiating low-grade and high-grade central chondral tumours. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2021.109579
Sharif B, Lindsay D, Saifuddin A (2022) Update on the imaging features of the enchondromatosis syndromes. Skeletal Radiol. https://doi.org/10.1007/s00256-021-03870-0
Davies AM, Patel A, James SL, Botchu R (2019) A retrospective validation of an imaging protocol for the management of solitary central cartilage tumours of the proximal humerus and around the knee. Clin Radiol. https://doi.org/10.1016/j.crad.2019.08.017
Shemesh SS, Acevedo-Nieves JD, Pretell-Mazzini J (2018) Treatment strategies for central low-grade chondrosarcoma of long bones: a systematic review of the literature and meta-analysis. Musculoskelet Surg. https://doi.org/10.1007/s12306-017-0507-7
Soldatos T, McCarthy EF, Attar S et al (2011) Imaging features of chondrosarcoma. J Comput Assist Tomogr. https://doi.org/10.1097/RCT.0b013e31822048ff
Sullivan CW, Kazley JM, Murtaza H et al (2020) Team approach: evaluation and management of low-grade cartilaginous lesions. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.19.00054
Parlier-Cuau C, Bousson V, Ogilvie CM et al (2011) When should we biopsy a solitary central cartilaginous tumor of long bones? Literature review and management proposal. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2010.06.051
Sampath Kumar V, Tyrrell PNM, Singh J et al (2016) Surveillance of intramedullary cartilage tumours in long bones. Bone Jt J. https://doi.org/10.1302/0301-620X.98B11.37864
Murphey MD, Flemming DJ, Boyea SR et al (1998) From the archives of the AFIP. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics.https://doi.org/10.1148/radiographics.18.5.9747616
Douis H, Singh L, Saifuddin A (2014) MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur Radiol. https://doi.org/10.1007/s00330-013-3003-y
Aoki J, Sone S, Fujioka F et al (1991) MR of enchondroma and chondrosarcoma. J Comput Assist Tomogr. https://doi.org/10.1097/00004728-199111000-00021
De Coninck T, Jans L, Sys G et al (2013) Dynamic contrast-enhanced MR imaging for differentiation between enchondroma and chondrosarcoma. Eur Radiol. https://doi.org/10.1007/s00330-013-2913-z
Lee FYI, Yu J, Chang SS et al (2004) Diagnostic value and limitations of fluorine-18 fluorodeoxyglucose positron emission tomography for cartilaginous tumors of bone. J Bone Jt Surg. https://doi.org/10.2106/00004623-200412000-00014
Subhawong TK, Winn A, Shemesh SS, Pretell-Mazzini J (2017) F-18 FDG PET differentiation of benign from malignant chondroid neoplasms: a systematic review of the literature. Skeletal Radiol. https://doi.org/10.1007/s00256-017-2685-7
Geirnaardt MUA, Hermans J, Bloem JL et al (1997) Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.169.4.9308471
Douis H, Parry M, Vaiyapuri S, Davies AM (2018) What are the differentiating clinical and MRI-features of enchondromas from low-grade chondrosarcomas? Eur Radiol. https://doi.org/10.1007/s00330-017-4947-0
Crim J, Schmidt R, Layfield L et al (2015) Can imaging criteria distinguish enchondroma from grade 1 chondrosarcoma? Eur J Radiol. https://doi.org/10.1016/j.ejrad.2015.06.033
Afonso PD, Isaac A, Villagrán JM (2019) Chondroid tumors as incidental findings and differential diagnosis between enchondromas and low-grade chondrosarcomas. Semin Musculoskelet Radiol. https://doi.org/10.1055/s-0038-1675550
Schumacher KM, Damron TA (2022) Evaluation of triage tool for low-grade cartilage tumors: Four-quadrant approach. J Surg Oncol. https://doi.org/10.1002/jso.26699
Deckers C, Steyvers MJ, Hannink G et al (2020) Can MRI differentiate between atypical cartilaginous tumors and high-grade chondrosarcoma? A systematic review. Acta Orthop. https://doi.org/10.1080/17453674.2020.1763717
Brien EW, Mirra JM, Luck J V (1999) Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. II. Juxtacortical cartilage tumors. Skeletal Radiol. https://doi.org/10.1007/s002560050466
Deckers C, Schreuder BHW, Hannink G et al (2016) Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J Surg Oncol. https://doi.org/10.1002/jso.24465
Herget GW, Kontny U, Saueressig U et al (2013) Osteochondrom und multiple Osteochondrome: Empfehlungen zur Diagnostik und Vorsorge unter besonderer Berücksichtigung des Auftretens sekundärer Chondrosarkome. Radiologe. https://doi.org/10.1007/s00117-013-2571-9
Vanel D, Kreshak J, Larousserie F et al (2013) Enchondroma vs. chondrosarcoma: a simple, easy-to-use, new magnetic resonance sign. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2011.11.043
Ferrer-Santacreu EM, Ortiz-Cruz EJ, González-López JM, Fernández EP (2012) Enchondroma versus low-grade chondrosarcoma in appendicular skeleton: clinical and radiological criteria. J Oncol. https://doi.org/10.1155/2012/437958
Logie C, Walker E, Forsberg J et al (2013) Chondrosarcoma: a diagnostic imager’s guide to decision making and patient management. Semin Musculoskelet Radiol. https://doi.org/10.1055/s-0033-1342967
Davies AM, Patel A, Botchu R et al (2021) The changing face of central chondrosarcoma of bone. One UK-based orthopaedic oncology unit’s experience of 33 years referrals. J Clin Orthop Trauma. https://doi.org/10.1016/j.jcot.2021.02.017
WHO Classification of Tumours Editorial Board and International Agency for Research on Cancer (2020) Soft Tissue and Bone Tumours - WHO Classification of Tumours (Medicine), 5th ed. ISBN: 978–92–832–4502–5
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. https://doi.org/10.1037/1040-3590.6.4.284
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. https://doi.org/10.1016/j.jcm.2016.02.012
Hwang S, Hameed M, Kransdorf M (2023) The 2020 World Health Organization classification of bone tumors: what radiologists should know. Skeletal Radiol. https://doi.org/10.1007/s00256-022-04093-7
Deckers C, van Zeijl NT, van Hooff ML et al (2023) Active surveillance of atypical cartilaginous tumours of bone: short term quality of life measurements. J Orthop Surg Res. https://doi.org/10.1186/s13018-023-03694-9
Mulligan ME (2019) How to diagnose enchondroma, bone infarct, and chondrosarcoma. Curr Probl Diagn Radiol. https://doi.org/10.1067/j.cpradiol.2018.04.002
Gassert FG, Breden S, Neumann J et al (2022) Differentiating enchondromas and atypical cartilaginous tumors in long bones with computed tomography and magnetic resonance imaging. Diagnostics. https://doi.org/10.3390/diagnostics12092186
Janzen L, Logan PM, O’Connell JX et al (1997) Intramedullary chondroid tumors of bone: Correlation of abnormal peritumoral marrow and soft-tissue MRI signal with tumor type. Skeletal Radiol. https://doi.org/10.1007/s002560050201
Douis H, Jeys L, Grimer R et al (2015) Is there a role for diffusion-weighted MRI (DWI) in the diagnosis of central cartilage tumors? Skeletal Radiol. https://doi.org/10.1007/s00256-015-2123-7
Rao A, Sharma C, Parampalli R (2019) Role of diffusion-weighted MRI in differentiating benign from malignant bone tumors. BJR Open. https://doi.org/10.1259/bjro.20180048
Jin B, Yang J, Zhen J et al (2023) Intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging can differentiate between atypical cartilaginous tumors and high-grade chondrosarcoma: correlation with histological vessel characteristics. J Comput Assist Tomogr. https://doi.org/10.1097/RCT.0000000000001515
Purandare NC, Puranik A, Shah S et al (2019) Can 18F-FDG PET/CT diagnose malignant change in benign chondroid tumors? Nucl Med Commun. https://doi.org/10.1097/MNM.0000000000001015
Gundavda MK, Agarwal MG, Singh N et al (2022) Can 18F-FDG PET/CT alone or combined with radiology be used to reliably grade cartilage bone neoplasms for surgical decision making? Nucl Med Commun. https://doi.org/10.1097/MNM.0000000000001498
Engel H, Herget GW, Füllgraf H et al (2021) Chondrogenic bone tumors: the importance of imaging characteristics. Rofo. https://doi.org/10.1055/a-1288-1209
Zhang Q, Xi Y, Li D et al (2020) The utility of 18F-FDG PET and PET/CT in the diagnosis and staging of chondrosarcoma: a meta-analysis. J Orthop Surg Res. https://doi.org/10.1186/s13018-020-01748-w
Annovazzi A, Anelli V, Zoccali C et al (2019) 18F-FDG PET/CT in the evaluation of cartilaginous bone neoplasms: the added value of tumor grading. Ann Nucl Med. https://doi.org/10.1007/s12149-019-01392-3
Johnson JD, Rainer WG, Rose PS, Houdek MT (2020) Utility of bone scintigraphy and PET-CT in the surgical staging of skeletal chondrosarcoma. Anticancer Res. https://doi.org/10.21873/anticanres.14588
Gitto S, Cuocolo R, van Langevelde K et al (2022) MRI radiomics-based machine learning classification of atypical cartilaginous tumour and grade II chondrosarcoma of long bones. EBioMedicine. https://doi.org/10.1016/j.ebiom.2021.103757
Gitto S, Cuocolo R, Albano D et al (2020) MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2020.109043
Gitto S, Cuocolo R, Annovazzi A et al (2021) CT radiomics-based machine learning classification of atypical cartilaginous tumours and appendicular chondrosarcomas. EBioMedicine. https://doi.org/10.1016/j.ebiom.2021.103407
Zhong J, Hu Y, Ge X et al (2023) A systematic review of radiomics in chondrosarcoma: assessment of study quality and clinical value needs handy tools. Eur Radiol. https://doi.org/10.1007/s00330-022-09060-3
Cilengir AH, Evrimler S, Serel TA et al (2023) The diagnostic value of magnetic resonance imaging-based texture analysis in differentiating enchondroma and chondrosarcoma. Skeletal Radiol. https://doi.org/10.1007/s00256-022-04242-y
Pan J, Zhang K, Le H et al (2021) Radiomics nomograms based on non-enhanced MRI and clinical risk factors for the differentiation of chondrosarcoma from enchondroma. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27690
Lisson CS, Lisson CG, Flosdorf K et al (2018) Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: a pilot study. Eur Radiol. https://doi.org/10.1007/s00330-017-5014-6
Eefting D, Schrage YM, Geirnaerdt MJA et al (2009) Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. https://doi.org/10.1097/PAS.0b013e31817eec2b
Acknowledgements
The authors would like to thank Heleen Coreelman (MSc in Biochemistry and Biotechnology, MSc in Forensic Science) for the editing of Fig. 1 and for proofreading the final version of the manuscript. They would also like to thank the pathology department of the Ghent University Hospital (Belgium) for their technical support and Griet Alleman (BSc Radiographer, Study Coordinator, Ghent University Hospital, Ghent, Belgium) for data collection and study management.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The senior scientific guarantor of this publication is the head of the research unit, Koenraad L. Verstraete (MD, PhD, Full Professor of Radiology).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors, Thomas Van Den Berghe (MD, PhD Researcher) and Esther Candries (MSc), have significant statistical expertise. Nevertheless, no complex statistical methods were necessary for this manuscript.
Informed consent
Written informed consent was waived by the Ethical Committee (Institutional Review Board) for this retrospective study involving human participants. For this type of study, formal consent is not required.
Ethical approval
Institutional Review Board approval was obtained (BC-08631 E01). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported in other articles.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Both first authors (T.V.D.B. and F.D.) contributed equally to this work and share first authorship status for this research paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Den Berghe, T., Delbare, F., Candries, E. et al. A retrospective external validation study of the Birmingham Atypical Cartilage Tumour Imaging Protocol (BACTIP) for the management of solitary central cartilage tumours of the proximal humerus and around the knee. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10604-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10604-y