Abstract
Objectives
Response evaluation criteria in solid tumors (RECIST) often fail to identify clinically meaningful response to bevacizumab-containing therapy in colorectal liver metastasis (CRLM). This study aimed to develop RECIST by combining contrast-enhanced and diffusion-weighted magnetic resonance imaging (MRI).
Methods
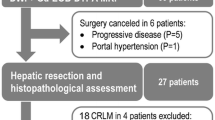
A total of 126 patients with CRLM who underwent hepatic resection after bevacizumab-containing chemotherapy were split into initial analyses cohort (N = 42, with 76 indexed liver metastases) and validation cohort (N = 84). In lesion-based analyses, percentage decrease of arterial enhancement area and percentage increase of apparent diffusion coefficient (ADC) value from baseline to post-chemotherapy were measured. Their optimal cutoff values for distinguishing pathology-confirmed major and minor response were determined. Then, the developed RECIST (D-RECIST) was established by combining functional and size-based items. Survival relevance of D-RECIST and RECIST was examined in the validation cohort.
Results
Percentage decrease of arterial enhancement area and increase of ADC value significantly differed between lesions of pathologic major or minor response, with optimal cutoffs of approximately 33% and 19%, respectively. Patients defined as responders by D-RECIST had a significantly longer median disease-free survival (DFS) than non-responders (p = 0.021; 12.9 versus 8.6 months). No significant difference was observed with RECIST (p = 0.524). In a Cox regression model, D-RECIST- but not RECIST-defined responses independently predicted the DFS (p = 0.034 and 0.811).
Conclusions
D-RECIST-defined responses provided significant prognostic information, and thus may serve as a better response evaluation approach than RECIST in CRLM treated with bevacizumab-containing therapy.
Key Points
• Changes in arterial enhancement area and apparent diffusion coefficient value are associated with pathological response in colorectal liver metastases treated with bevacizumab.
• The MRI-based response criteria developed by combining size-based and functional features can provide significant prognostic information.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CRLM:
-
Colorectal liver metastasis
- DFS:
-
Disease-free survival
- DWI:
-
Diffusion-weighted imaging
- RECIST:
-
Response evaluation criteria in solid tumors
- ROC:
-
Receiver-operating characteristic
- ROI:
-
Region of interest
- T1WI:
-
T1-weighted imaging
References
Al-Asfoor A, Fedorowicz Z, Lodge M (2008) Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006039.pub4
Martin RC 2nd, Scoggins CR, Schreeder M et al (2015) Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 121:3649–3658
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Gerwing M, Herrmann K, Helfen A et al (2019) The beginning of the end for conventional RECIST - novel therapies require novel imaging approaches. Nat Rev Clin Oncol 16:442–458
Krajewski KM, Nishino M, Franchetti Y, Ramaiya NH, Van den Abbeele AD, Choueiri TK (2014) Intraobserver and interobserver variability in computed tomography size and attenuation measurements in patients with renal cell carcinoma receiving antiangiogenic therapy: implications for alternative response criteria. Cancer 120:711–721
Ma X, Phi Van V, Kimm MA et al (2017) Integrin-targeted hybrid fluorescence molecular tomography/X-ray computed tomography for imaging tumor progression and early response in non-small cell lung cancer. Neoplasia 19:8–16
Chun YS, Vauthey JN, Boonsirikamchai P et al (2009) Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 302:2338–2344
Shindoh J, Loyer EM, Kopetz S et al (2012) Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 30:4566–4572
Nishioka Y, Shindoh J, Yoshioka R et al (2015) Radiological morphology of colorectal liver metastases after preoperative chemotherapy predicts tumor viability and postoperative outcomes. J Gastrointest Surg 19:1653–1661
Mazard T, Boonsirikamchai P, Overman MJ et al (2018) Comparison of early radiological predictors of outcome in patients with colorectal cancer with unresectable hepatic metastases treated with bevacizumab. Gut 67:1095–1102
Ward J, Robinson PJ, Guthrie JA et al (2005) Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology 237:170–180
Zech CJ, Korpraphong P, Huppertz A et al (2014) Randomized multicentre trial of gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg 101:613–621
van Kessel CS, Buckens CF, van den Bosch MA, van Leeuwen MS, van Hillegersberg R, Verkooijen HM (2012) Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol 19:2805–2813
Kulemann V, Schima W, Tamandl D et al (2011) Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol 79:e1–e6. https://doi.org/10.1016/j.ejrad.2010.03.004
Schulz A, Viktil E, Godt JC et al (2016) Diagnostic performance of CT, MRI and PET/CT in patients with suspected colorectal liver metastases: the superiority of MRI. Acta Radiol 57:1040–1048
Sivesgaard K, Larsen LP, Sørensen M et al (2018) Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol 28:4735–4747
Barabasch A, Heinzel A, Bruners P, Kraemer NA, Kuhl CK (2018) Diffusion-weighted MRI is superior to PET/CT in predicting survival of patients undergoing (90)Y radioembolization of hepatic metastases. Radiology 288:764–773
Kannan P, Kretzschmar WW, Winter H et al (2018) Functional parameters derived from magnetic resonance imaging reflect vascular morphology in preclinical tumors and in human liver metastases. Clin Cancer Res 24:4694–4704
Heijmen L, Ter Voert EE, Nagtegaal ID et al (2013) Diffusion-weighted MR imaging in liver metastases of colorectal cancer: reproducibility and biological validation. Eur Radiol 23:748–756
Fouladi DF, Zarghampour M, Pandey P et al (2020) Baseline 3D-ADC outperforms 2D-ADC in predicting response to treatment in patients with colorectal liver metastases. Eur Radiol 30:291–300
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Blazer DG 3rd, Kishi Y, Maru DM et al (2008) Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 26:5344–5351
Miles KA, Leggett DA, Kelley BB, Hayball MP, Sinnatamby R, Bunce I (1998) In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol 71:276–281
Hackl C, Schacherer D, Anders M et al (2016) Improved detection of preclinical colorectal liver metastases by high resolution ultrasound including molecular ultrasound imaging using the targeted contrast agent BR55. Ultraschall Med 37:290–296
Edrei Y, Gross E, Corchia N et al (2011) Vascular profile characterization of liver tumors by magnetic resonance imaging using hemodynamic response imaging in mice. Neoplasia 13:244–253
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Litiere S, Isaac G, De Vries EGE et al (2019) RECIST 1.1 for response evaluation apply not only to chemotherapy-treated patients but also to targeted cancer agents: a pooled database analysis. J Clin Oncol 37:1102–1110
Cabanillas ME, de Souza JA, Geyer S et al (2017) Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: results of a multicenter phase II International Thyroid Oncology Group Trial. J Clin Oncol 35:3315–3321
De Bruyne S, Van Damme N, Smeets P et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106:1926–1933
Wong GYM, Kumar R, Beeke C et al (2018) Survival outcomes for patients with indeterminate 18FDG-PET scan for extrahepatic disease before liver resection for metastatic colorectal cancer: a retrospective cohort study using a prospectively maintained database to analyze survival outcomes for patients with indeterminate extrahepatic disease on 18FDG-PET scan before liver resection for metastatic colorectal cancer. Ann Surg 267:929–935
Funding
This study has received funding by the National Natural Science Foundation of China (No. 81701656).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Meng-Su Zeng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li-Heng Liu and Guo-Feng Zhou shared co-first authorship.
Rights and permissions
About this article
Cite this article
Liu, LH., Zhou, GF., Lv, H. et al. Identifying response in colorectal liver metastases treated with bevacizumab: development of RECIST by combining contrast-enhanced and diffusion-weighted MRI. Eur Radiol 31, 5640–5649 (2021). https://doi.org/10.1007/s00330-020-07647-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07647-2