Abstract
Objectives
Patients with haemoptysis often experience daily physical and mental impairment. Bronchial artery embolisation is among the first-line treatment options used worldwide; however, no evidence exists regarding the health-related quality of life (HRQoL) after bronchial artery embolisation. Therefore, this study aimed to evaluate the effects of bronchial artery embolisation on the HRQoL of patients with haemoptysis.
Methods
We prospectively enrolled 61 consecutive patients who visited our hospital from July 2017 to August 2018 and received bronchial artery embolisation for haemoptysis. The primary outcome was the HRQoL evaluated using the Short Form Health Survey, which contains physical and mental components, before and after bronchial artery embolisation. The secondary outcomes were procedural success, complications, and recurrence-free survival rate at 6 months.
Results
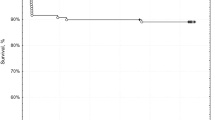
The mean age of the patients was 69 years (range, 31–87 years). The procedural success rate was 98%. No major complications occurred. The recurrence-free survival rate estimated using the Kaplan-Meier analysis at 6 months after bronchial artery embolisation was 91.8% (95% confidence interval, 91.1–92.5%). Compared with the pre-treatment scores, the physical and mental scores were significantly improved at 6 months after bronchial artery embolisation (p < 0.05).
Conclusion
Bronchial artery embolisation improved the HRQoL of patients with haemoptysis.
Key Points
• Bronchial artery embolisation improved the HRQoL of patients with haemoptysis.
• Vessel dilation on computed tomography and systemic artery-pulmonary artery direct shunting on angiography were the most common abnormalities.
• The recurrence-free survival rate estimated using the Kaplan-Meier analysis at 6 months after bronchial artery embolisation was 91.8%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemoptysis is a potentially life-threatening condition with mortality rates ranging from 7 to 30% for patients with massive haemoptysis [1, 2]. Previously, surgical management was the only available definitive therapy for life-threatening haemoptysis. However, given the higher risks associated with surgery, initial intervention with bronchial artery embolisation (BAE) is currently a preferred alternative and one of the first-line options for haemoptysis [3, 4]. BAE, which uses a bronchial-pulmonary artery shunting mechanism, is an effective therapy for moderate to severe haemoptysis [5, 6]; however, it is associated with several complications, including chest pain, fever, mediastinal haematoma, and aortic dissection. Additionally, post-BAE recurrence rates have been reported to be as high as 10–57.5% [5,6,7,8].
Health-related quality of life (HRQoL) is defined as an individual’s overall satisfaction with life and functional status in relation to disease [9]. Haemoptysis often occurs suddenly and without any prodromal symptoms, such as haemo-sputum [5, 10]. Furthermore, patients with haemoptysis experience both acute and chronic decreases in terms of HRQoL [5, 11, 12]. They typically experience anticipatory anxiety related to their fear of death, which is often strengthened by the sudden onset of haemoptysis and restricted physical activity [3, 11]. Patients with severe cases may develop neurosis due to the fear of suffocation and death; however, BAE can potentially improve their HRQoL dramatically [13]. Therefore, it is necessary to determine the measurable post-BAE improvements in HRQoL.
To our knowledge, no study has investigated the effects of BAE on measurable HRQoL. Therefore, we evaluated the effects of BAE on the HRQoL of patients with haemoptysis.
Materials and methods
Patient population
We prospectively enrolled all patients who visited the Hemoptysis and Pulmonary-Circulation Center at Eishinkai Kishiwada Rehabilitation Hospital from July 2017 to August 2018, and underwent BAE for haemoptysis. We did not treat haemoptysis caused by lung cancer because previous related studies of BAE were limited by their sample size; moreover, its efficacy and safety remain unclear [14,15,16]. Our BAE candidates were patients diagnosed with haemoptysis with a volume ≥ 20 mL/day and worsened quality of life. We defined haemoptysis < 39 mL/day, 40–199 mL/day, and ≥ 200 mL/day as mild, moderate, and massive, respectively [4, 5, 12]. This study was approved by the institutional review board of Eishinkai Kishiwada Rehabilitation Hospital (approval date, October 13, 2016; approval number, 2016-001). Written informed consent was obtained through the questionnaires.
Imaging study interpretation
All the patients underwent pre-BAE CT angiography with contrast medium enhancement to evaluate the baseline disease, bleeding site, and possible haemoptysis-related arteries (HRAs) for procedural planning. CT findings of possible HRAs and bleeding were as follows: vessel dilation, aneurysmal formation, direct vessel shunting, ground-glass attenuation suggesting inhaled blood, tortuosity, hypervascularity, and enhancement of distal part of the HRAs embolised at prior BAE [5, 7, 12]. Three-dimensional images were reconstructed using Ziostation 2 version 2.9.2.2 CT (Canon Aquilion Lightning 80).
BAE procedure
Trained pulmonologists specialising in the treatment of pulmonary arterio-venous malformations and BAE performed all angiography and embolisation procedures. BAE for aorta-related HRAs was performed using the trans-femoral artery approach, whereas subclavian-related and axillary-related HRAs were performed using the trans-radial or trans-brachial artery approach. The number and approach sites for BAE were determined at the discretion of the attending physician while considering the CT-suggested possible HRAs. All possible CT-defined HRAs were evaluated by selective angiography using a 4-Fr or 5-Fr guiding catheter. In the case of abnormal findings, including systemic artery-pulmonary artery direct shunting and hypervascularity, HRAs were selectively embolised using the 3-Fr microcatheter system with 0.014-in. or 0.016-in. guide wires. Metallic platinum coils (detachable or pushable coils; IDC®, Boston Scientific; Target®, Stryker; Azur®, Terumo Corporation; and C-STOPPER®, PIOLAX Inc.) were used as the embolic agents.
Procedural success was defined as complete HRAs embolisation. We defined the termination of all HRAs embolisation, including its failure, as one series. BAE-naive patients were classified as the initial treatment subgroup. Patients who had previously undergone BAE at least once were classified as the recurrence subgroup. Recurrence events during this study period were classified as haemoptysis ≥ 20 mL/day requiring BAE re-treatment or death due to haemoptysis. If haemoptysis recurred, then repeat BAE was planned.
Complications
We used intra-procedural and post-procedural observations to determine early BAE complications. Complications were defined using the criteria proposed by the Society of Interventional Radiology Standards of Practice Committee [17].
Outcome measures
We investigated baseline characteristics, smoking status, comorbidities, baseline pulmonary diseases, modified Medical Research Council scores, hospital stay lengths, procedural success, complications, post-BAE recurrence-free survival rate at 6 months, patient satisfaction with treatment, and HRQoL.
The HRQoL survey was performed as a self-assessment by patients themselves using the SF-8, which is a shortened version of the SF-36 health survey. The SF-8 is the most popular assessment tool, and it is widely used for evaluating HRQoL related to many diseases [18, 19]. Previous studies have confirmed the validity and reliability of the Japanese version of the SF-8 [20, 21]. The raw scores of answers to each SF-8 question were converted to a scale of 0–100, with scores of 0 and 100 indicating the worst and best life quality, respectively. Notably, the reported mean SF-8 score for each summary parameter in the Japanese general population was 50 points. Therefore, all SF-8 assessments were compared with the average score of the general Japanese population [21].
To calculate comparable scores, the SF-8 evaluates eight items regarding health status. These include four physical (physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH)) and four mental (vitality (VT), social functioning (SF), mental health (MH), role emotional (RE)) components. The physical and mental component scores were used to calculate summary measures termed the physical component score (PCS) and mental component score (MCS), respectively [22]. SF-8 scores were obtained using the questionnaire assessment results before treatment and at 1, 3, and 6 months after BAE. Quantitative variables were expressed using mean and standard deviation (SD) values. We calculated the percentage of the change in the score at 6 months compared to the baseline score. All modified Medical Research Council scores were obtained before BAE treatment.
Moreover, we investigated the self-assessed subjective satisfaction with treatment at 6 months after BAE by using the following scale: very satisfied, satisfied, neither satisfied nor dissatisfied, somewhat dissatisfied, or dissatisfied.
Statistical analysis
The Wilcoxon signed-rank test was used to compare the baseline SF-8 scores with those at 1, 3, and 6 months after BAE. The Mann-Whitney test and chi-squared test were used to compare baseline characteristics of the initial treatment and recurrence groups. The Kaplan-Meier analysis was used to estimate the haemoptysis-free recurrence rate at 6 months after BAE. Statistical significance was defined as p < 0.05. All analyses were performed using the statistical package SPSS (SPSS version 22; SPSS).
Results
Patient characteristics and clinical outcomes
We excluded eight patients who were lost to follow-up among a total of 69 with haemoptysis who visited the Hemoptysis and Pulmonary-Circulation Center (EHPC) at Eishinkai Kishiwada Rehabilitation Hospital between July 2017 and August 2018. Finally, we enrolled 61 patients with available data who underwent BAE. Table 1 summarises the characteristics of the patients and BAE procedural information. The mean age of patients was 69 years (range, 31–87 years), and 24 (39%) patients were male.
There were 14 (20%) and 39 (64%) patients with moderate and massive haemoptysis, respectively. Regarding the BAE series, the initial and recurrence treatment subgroups comprised 37 (61%) and 24 (39%) patients, respectively. The mean hospital length of stay was 5.8 days (95% confidence interval (CI), 4.6–7.1). There were no major complications and nine cases of minor complications: mediastinal haematoma, 1 case; chest pain, 1 case; back pain, 1 case; vomiting, 2 cases; extravasation, 2 cases; and vagal reflex, 2 cases. The recurrence-free survival rate at 6 months was 91.8% (95% CI, 91.1–92.5%). In the recurrence treatment subgroup, baseline diseases were bronchiectasis/cystic fibrosis (10 cases), non-tuberculous mycobacteria (8 cases), pulmonary aspergillosis (3 cases), cryptogenic haemoptysis (2 cases), and pulmonary tuberculous sequelae (1 case). The mean haemoptysis-free interval for this recurrence subgroup was 606 days (95% CI, 415–797 days). Five patients in the recurrent subgroup presented recurrence events during the study period. Three patients underwent repeat BAE uneventfully and haemostasis was achieved. One patient with non-tuberculous mycobacteria (NTM) was treated with repeat BAE; however, haemoptysis continued. Moreover, surgical resection was discouraged due to diffuse pulmonary disease, and the patient died because of respiratory failure under mechanical ventilation support 1 week after the initial BAE treatment. Another patient with haemoptysis caused by aspergillosis died because of respiratory failure secondary to bacterial pneumonia at 3 months after BAE.
Table 2 summarises the CT findings, angiographic findings, and HRA information. The most common CT findings were vessel dilation (51%) and tortuosity (26%). The most common angiographic findings were systemic artery-pulmonary artery direct shunting (57%) and vessel dilation (54%). Regarding HRAs, the bronchial artery (44%) was the most common artery, followed by the intercostal artery (18%). Figure 1 shows three-dimensional CT and angiography findings of vessel dilation, tortuosity, hypervascularity, and systemic artery-pulmonary artery direct shunting.
Three-dimensional CT (a, c) and angiography (b, d) findings before BAE. a Abnormal pulmonary ligament artery with vessel dilation (white arrows) and tortuosity (black arrows). b Vessel dilation (white arrows), tortuosity (black arrows), and systemic artery-pulmonary artery direct shunting (red arrows). c Abnormal bronchial artery with hypervascularity (white arrows). d Hypervascularity (white arrows) and systemic artery-pulmonary artery direct shunting (black arrows)
Quality of life analysis
Table 3 shows the individual SF-8 scores before treatment and at 1, 3, and 6 months after BAE. The SF-8 questionnaire was completed by all 61 patients before treatment. Two patients died within 1 month and 3 months, respectively, after BAE; therefore, their subsequent assessments were unavailable. One patient and three patients did not return the questionnaire at 1 month and 3 months, respectively. Among the treated patients, the physical (PCS, PF, RP, GH) and mental (MCS, VT, RE, MH) component scores at 1 month were significantly better than those before treatment (p < 0.05). At 3 months, all scores except those for BP and SF were significantly better than those before treatment (p < 0.05). At 6 months, the physical (PF, GH) and mental (MCS, VT, RE, MH) component scores were better than those before treatment (p < 0.05). Compared with the pre-treatment scores, the scores for PF, GH, and all mental components improved by > 10%, whereas those for the remaining physical components (PCS, RP, and BP) improved at 6 months after treatment (by 9.1%, 7.7%, and 9.3%, respectively).
Figure 2 visually represents SF-8 score changes from before treatment to 6 months after BAE for the physical and mental components. There was no difference in the baseline characteristics of the initial and recurrence treatment subgroups (Table 4). Compared with the pre-treatment scores, the initial treatment subgroup showed a significant improvement in GH and all mental components at 6 months after treatment (Table 5). Compared with the pre-treatment scores, PF in the recurrence subgroup improved at 6 months (Table 6).
Self-assessed satisfaction grades were obtained from 58 patients: 45% were very satisfied (26/58 patients); 48% were satisfied (28/58 patients); 7% were neither satisfied nor dissatisfied (4/58 patients); and none was somewhat dissatisfied or dissatisfied.
Discussion
This is the first study to demonstrate the improvement in the HRQoL of patients with haemoptysis treated with BAE. The main findings were that BAE was beneficial for improving the HRQoL of patients with haemoptysis, particularly the mental impairment scores of the initial treatment subgroup, and that the efficacy and safety of BAE could contribute to HRQoL improvement.
We observed significant improvements in the physical (PF, GH) and mental (MCS, VT, RE, MH) component scores at 6 months after BAE compared with the pre-treatment scores of the entire treated population.
Both the SF-8 and SF-36 scores varied according to disease stage, severity, and status, even for patients with similar pulmonary diseases [14, 23]; therefore, the efficacy of the treatment in terms of HRQoL should be evaluated based on the changes and/or relative values compared with the average score of 50 for the general population. However, there is no generally accepted definition of a minimally important change in the quality of life [24]; a 10% difference in the SF-36 summary scores is generally considered a clinically relevant difference [25].
Most mental scores showed a significant improvement at 1 month after treatment, and this improvement was maintained for 6 months. All mental score improvement ratios exceeded 10% within 6 months, with > 90% of the patients reporting satisfaction with the treatment. Given the favourable improvement in mental impairment, we assumed that the patients experienced increased confidence that haemoptysis might not occur suddenly and place them at risk for more severe health outcomes. These results indicate that BAE notably affected the psychological wellness of patients.
Conversely, although most physical component scores improved at 1 month and 3 months, there was a modest decline in scores at 6 months compared with scores at 3 months and in the improvement trend compared with the pre-treatment values. The modest decline in the physical scores at 6 months could be attributed to the following factors:
-
1.
Haemoptysis recurrence: Five patients experienced recurrence during this study period. Recurrent events during the study period and the presence of haemo-sputum and mild haemoptysis not requiring repeat BAE might have affected the physical status of the patients.
-
2.
Worsening of baseline pulmonary diseases: Baseline pulmonary diseases, including NTM disease and pulmonary aspergillosis, have slowly progressing clinical courses [26, 27] (i.e., the 5-year mortality rates varied between 5 and 42% for NTM [26] and between 38 and 85% for chronic pulmonary aspergillosis [28]).
-
3.
Comorbidities: We enrolled elderly individuals with widely varying comorbidities, including stroke, coronary heart disease, malignancy, and connective tissue disease.
Because BAE only controls haemoptysis and not baseline diseases, we considered physical improvements in the HRQoL to be the result of haemostasis induced by BAE at 1 month and 3 months. Moreover, the aforementioned factors are associated, individually or in combination, with a modest subsequent physical decline in the clinical overall outcomes of patients.
Regarding the subgroup analysis, the initial treatment subgroup showed improvements in GH and all mental components at 6 months. These subgroup analyses yielded results similar to those of the entire population; however, in the recurrence subgroup, only PF showed an improvement at 6 months. We previously reported that the procedural success rate of repeat BAE was 97.7% [12]; therefore, repeat haemoptysis can be technically treated using BAE. Nevertheless, all recurrences and death events occurred in the recurrence subgroup. The subgroup analysis showed that BAE resulted in HRQoL improvements in the initial treatment subgroup.
Previous studies reported BAE complications involving vascular injuries, such as vasospasm, aortic dissection, and perforation (0.3–13%) [22, 29,30,31,32,33,34], and spinal cord ischaemia that led to transient or permanent paraplegia (0.6–4.4%) [8, 35, 36]. There are various types of embolic agents, and complication rates differ across agents. Regarding BAE using a metallic coil, a previous study reported that haemostasis rates were > 90% at 1 year, which are comparable to those for other embolic agents, and that major complication rates were favourable (0–1.4%) [5, 6, 12, 37]. Our findings support the efficacy and safety of BAE using a metallic coil; moreover, the lower invasiveness of BAE was thought to have contributed to the improvement in HRQoL.
This study had several limitations. First, the sample size was small, which limited its statistical power. Second, we did not treat haemoptysis caused by lung cancer; therefore, our findings cannot be applied to patients with lung cancer. We evaluated the HRQoL of patients with haemoptysis, but the study period was short; therefore, we did not evaluate the long-term status of HRQoL improvements after BAE. Because N-butyl-2-cyanoacrylate and polyvinyl alcohol are not covered by Japanese public health insurance, we used a metallic coil as the embolic agent. Therefore, it is unclear whether these findings could be applied to other embolic agents. Because two patients died during the study period, their responses could not be obtained. Therefore, the results could have been worse if their responses were included. Moreover, we did not assess the standard of living, educational level, economic status, and family issues, which affect the physical and mental status of patients. Nevertheless, our results indicate that BAE improved the HRQoL of patients with haemoptysis.
Abbreviations
- BAE:
-
Bronchial artery embolisation
- BP:
-
Bodily pain
- COPD:
-
Chronic obstructive pulmonary disease
- GH:
-
General health
- HRAs:
-
Haemoptysis-related arteries
- HRQoL:
-
Health-related quality of life
- MCS:
-
Mental component score
- MH:
-
Mental health
- NTM:
-
Non-tuberculous mycobacteria
- PCS:
-
Physical component score
- RE:
-
Role emotional
- RP:
-
Role physical
- SD:
-
Standard deviation
- SF:
-
Social functioning
- VT:
-
Vitality
References
Fartoukh M, Khoshnood B, Parrot A et al (2012) Early prediction of in-hospital mortality of patients with hemoptysis: an approach to defining severe hemoptysis. Respiration 83:106–114. https://doi.org/10.1159/000331501
Ong TH, Eng P (2003) Massive hemoptysis requiring intensive care. Intensive Care Med 29:317–320. https://doi.org/10.1007/s00134-002-1553-6
Davidson K, Shojaee S (2020) Managing massive hemoptysis. Chest 157:77–88
Hanley M, Ahmed O, Chandra A et al (2016) ACR appropriateness criteria clinically suspected pulmonary arteriovenous malformation. J Am Coll Radiol 13:796–800. https://doi.org/10.1016/j.jacr.2016.03.020
Ishikawa H, Hara M, Ryuge M et al (2017) Efficacy and safety of super selective bronchial artery coil embolisation for haemoptysis: a single-centre retrospective observational study. BMJ Open 7:e014805. https://doi.org/10.1136/bmjopen-2016-014805
Woo S, Yoon CJ, Chung JW et al (2013) Bronchial artery embolisation to control hemoptysis: comparison of n-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology 269:594–602. https://doi.org/10.1148/radiol.13130046
Panda A, Bhalla AS, Goyal A (2017) Bronchial artery embolisation in hemoptysis: a systematic review. Diagn Interv Radiol 23:307–317. https://doi.org/10.5152/dir.2017.16454
Fruchter O, Schneer S, Rusanov V, Belenky A, Kramer MR (2015) Bronchial artery embolisation for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann 23:55–60. https://doi.org/10.1177/0218492314544310
Karimi M, Brazier J (2016) Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics 34:645–649. https://doi.org/10.1007/s40273-016-0389-9
Ogata H, Matsumoto K, Shinozaki S et al (2019) Meteorological effects on severe hemoptysis: a hospital-based observational study. Respir Investig 57:361–367. https://doi.org/10.1016/j.resinv.2019.02.005
Romàn CM, Loughlin HC, Aliaj E, Fay RJ, Tran QT, Borowitz D (2020) Hemoptysis from the perspective of people with cystic fibrosis. Clin Respir J 14:299–303. https://doi.org/10.1111/crj.13132
Ryuge M, Hara M, Hiroe T et al (2019) Mechanisms of recurrent haemoptysis after super-selective bronchial artery coil embolisation: a single-centre retrospective observational study. Eur Radiol 29:707–715. https://doi.org/10.1007/s00330-018-5637-2
Lee MK, Kim S-H, Yong SJ et al (2015) Moderate hemoptysis: recurrent hemoptysis and mortality according to bronchial artery embolisation. Clin Respir J 9:53–64. https://doi.org/10.1111/crj.12104
Mehta M, Marras TK (2011) Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med 105:1718–1725. https://doi.org/10.1016/j.rmed.2011.08.004
Witt C, Schmidt B, Geisler A et al (2000) Value of bronchial artery embolisation with platinum coils in tumorous pulmonary bleeding. Eur J Cancer 36:1949–1954. https://doi.org/10.1016/s0959-8049(00)00188-x
Park HS, Il KY, Kim HY, Zo JI, Lee JH, Lee JS (2007) Bronchial artery and systemic artery embolisation in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol 30:638–643. https://doi.org/10.1007/s00270-007-9034-5
Angle JF, Siddiqi NH, Wallace MJ et al (2010) Quality improvement guidelines for percutaneous transcatheter embolisation: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 21:1479–1486. https://doi.org/10.1016/j.jvir.2010.06.014
Crins MHP, van der Wees PJ, Klausch T, van Dulmen SA, Roorda LD, Terwee CB (2018) Psychometric properties of the PROMIS Physical Function item bank in patients receiving physical therapy. PLoS One 13:e0192187. https://doi.org/10.1371/journal.pone.0192187
Ricotti S, Martinelli V, Caspani P et al (2017) Changes in quality of life and functional capacity after lung transplantation: a single-center experience. Monaldi Arch Chest Dis 87:831. https://doi.org/10.4081/monaldi.2017.831
Vallès J, Guilera M, Briones Z et al (2010) Validity of the Spanish 8-item short-form generic health-related quality-of-life questionnaire in surgical patients. Anesthesiology 112:1164–1174. https://doi.org/10.1097/ALN.0b013e3181d3e017
Fukuhara S, Suzukamo Y (2004) Manual of the SF-8 Japanese version. Institute for Health & Process Evaluation Research, Kyoto
Shao H, Wu J, Wu Q et al (2015) Bronchial artery embolisation for hemoptysis: a retrospective observational study of 344 patients. Chin Med J (Engl) 128:58–62. https://doi.org/10.4103/0366-6999.147811
Kamata H, Asakura T, Suzuki S et al (2017) Impact of chronic Pseudomonas aeruginosa infection on health-related quality of life in Mycobacterium avium complex lung disease. BMC Pulm Med 17:198. https://doi.org/10.1186/s12890-017-0544-x
Wyrwich KW, Bullinger M, Aaronson N et al (2005) Estimating clinically significant differences in quality of life outcomes. Qual Life Res 14:285–295
Möller A, Sartipy U (2012) Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol 7:183–187. https://doi.org/10.1097/JTO.0b013e3182340abb
Diel R, Lipman M, Hoefsloot W (2018) High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis 18:206. https://doi.org/10.1186/s12879-018-3113-x
Quint JK, Smith MP (2019) Paediatric and adult bronchiectasis: diagnosis, disease burden and prognosis. Respirology 24:413–422. https://doi.org/10.1111/resp.13495
Lowes D, Al-Shair K, Newton PJ et al (2017) Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 49:1601062. https://doi.org/10.1183/13993003.01062-2016
Bhalla A, Kandasamy D, Veedu P, Mohan A, Gamanagatti S (2015) A retrospective analysis of 334 cases of hemoptysis treated by bronchial artery embolisation. Oman Med J 30:119–128. https://doi.org/10.5001/omj.2015.26
Tom LM, Palevsky HI, Holsclaw DS et al (2015) Recurrent bleeding, survival, and longitudinal pulmonary function following bronchial artery embolisation for hemoptysis in a U.S. adult population. J Vasc Interv Radiol 26:1806-1813.e1. https://doi.org/10.1016/j.jvir.2015.08.019
Swanson KL, Johnson CM, Prakash UBS, McKusick MA, Andrews JC, Stanson AW (2002) Bronchial artery embolisation: experience with 54 patients. Chest 121:789–795
Chun J-Y, Belli A-M (2010) Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 20:558–565. https://doi.org/10.1007/s00330-009-1591-3
Shin BS, Jeon GS, Lee SA, Park M-H (2011) Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 15:1093–1098. https://doi.org/10.5588/ijtld.10.0659
Yoo DH, Yoon CJ, Kang S-G, Burke CT, Lee JH, Lee C-T (2011) Bronchial and nonbronchial systemic artery embolisation in patients with major hemoptysis: safety and efficacy of n-butyl cyanoacrylate. AJR Am J Roentgenol 196:W199–W204. https://doi.org/10.2214/AJR.10.4763
Rémy J, Arnaud A, Fardou H, Giraud R, Voisin C (1977) Treatment of hemoptysis by embolisation of bronchial arteries. Radiology 122:33–37. https://doi.org/10.1148/122.1.33
Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL (1996) Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolisation. Radiology 200:691–694. https://doi.org/10.1148/radiology.200.3.8756916
Ando T, Kawashima M, Masuda K et al (2017) Clinical and angiographic characteristics of 35 patients with cryptogenic hemoptysis. Chest 152:1008–1014. https://doi.org/10.1016/j.chest.2017.05.007
Acknowledgements
We express our gratitude to Dr. Shigeo Shirakawa (president of Seiwakai Hospital Group), Mr. Unshow Shirakawa (vice president of Seiwakai Hospital Group), and the Japan Society of Clinical Research for their dedicated support of our work.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
N.O. and H.I. had full access to the data and take responsibility for its integrity and have final responsibility for the decision to submit for publication. M.H. and S.Y. are responsible for the study concept and design, design analysis, and interpretation of data. T.N., Y.Y., Y.Y., M.Y., T.H., and K.K. are responsible for acquisition of data. T.K. and M.F. are responsible for critical revision of the manuscript.
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Naoki Omachi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Masahiko Hara kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omachi, N., Ishikawa, H., Hara, M. et al. The impact of bronchial artery embolisation on the quality of life of patients with haemoptysis: a prospective observational study. Eur Radiol 31, 5351–5360 (2021). https://doi.org/10.1007/s00330-020-07533-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07533-x