Abstract
Objectives
This prospective trial was performed to verify whether microwave ablation (MWA) in combination with chemotherapy could provide superior survival benefit compared with chemotherapy alone.
Materials and methods
From March 1, 2015, to June 20, 2017, treatment-naïve patients with pathologically verified advanced or recurrent non-small cell lung cancer (NSCLC) were randomly assigned to MWA plus chemotherapy group or chemotherapy group. The primary endpoint was progression-free survival (PFS), while the secondary endpoints included overall survival (OS), time to local progression (TTLP), and objective response rate (ORR). The complications and adverse events were also reported.
Results
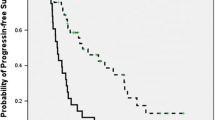
A total of 293 patients were randomly assigned into the two groups. One hundred forty-eight patients with 117 stage IV tumors were included in the MWA plus chemotherapy group. One hundred forty-five patients with 113 stage IV tumors were included in the chemotherapy group. The median follow-up period was 13.1 months and 12.4 months, respectively. Median PFS was 10.3 months (95% CI 8.0–13.0) in the MWA plus chemotherapy group and 4.9 months (95% CI 4.2–5.7) in the chemotherapy group (HR = 0.44, 95% CI 0.28–0.53; p < 0.0001). Median OS was not reached in the MWA plus chemotherapy group and 12.6 months (95% CI 10.6–14.6) in the chemotherapy group (HR = 0.38, 95% CI 0.27–0.53; p < 0.0001) using Kaplan-Meier analyses with log-rank test. The median TTLP was 24.5 months, and the ORR was 32% in both groups. The adverse event rate was not significantly different in the two groups.
Conclusions
In patients with advanced NSCLC, longer PFS and OS can be achieved with the treatment of combined MWA and chemotherapy than chemotherapy alone.
Key Points
• Patients treated with MWA plus chemotherapy had superior PFS and OS over those treated with chemotherapy alone.
• The ORR of patients treated with MWA plus chemotherapy was similar to that of those treated with chemotherapy alone.
• Complications associated with MWA were common but tolerable and manageable.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confident interval
- CTCAE:
-
Common Terminology Criteria Adverse Events Version
- DCR:
-
Disease control rate
- ECOG:
-
Eastern Cooperation Oncology Group
- EGFR:
-
Epidermal growth factor receptor
- EML4-ALK:
-
Echinoderm microtubule–associated protein-like 4-anaplastic lymphoma kinase
- GGO:
-
Ground glass opacity
- HR:
-
Hazard ratio
- MWA:
-
Microwave ablation
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-L1:
-
Program death-ligand 1
- PFS:
-
Progression-free survival
- PS:
-
Performance status
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SIR:
-
Society of Interventional Radiology
- TKIs:
-
Tyrosine kinase inhibitors
- TTLP:
-
Time to local progression
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Chen W, Zheng R, Zhang S et al (2017) Cancer incidence and mortality in China, 2013. Cancer Lett 401:63–71
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177
Peters S, Camidge DR, Shaw AT et al (2017) Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829–838
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Schiller JH, Harrington D, Belani CP et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Esteban E, Casillas M, Cassinello A (2009) Pemetrexed in first-line treatment of non-small cell lung cancer. Cancer Treat Rev 35:364–373
Yu J, Liang P, Yu XL et al (2012) US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263(3):900–908
Song Z, Qi H, Zhang H et al (2017) Microwave ablation: results with three different diameters of antennas in bovine and porcine liver. J Cancer Res Ther 13:737–741
Klapperich ME, Abel EJ, Ziemlewicz TJ et al (2017) Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology 284:272–280
Groeschl RT, Pilgrim CH, Hanna EM et al (2014) Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 259:1195–1200
Lau WY, Leung TW, Yu SC, Ho SK (2003) Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg 237:171–179
Lencioni R (2010) Loco-regional treatment of hepatocellular carcinoma. Hepatology 52:762–773
Liapi E, Geschwind JF (2007) Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol 25:978–986
Dupuy DE (2011) Image-guided thermal ablation of lung malignancies. Radiology 260:633–655
Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE (2011) Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–651
Yang X, Ye X, Zheng A et al (2014) Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 110:758–763
Yang X, Ye X, Huang G et al (2017) Repeated percutaneous microwave ablation for local recurrence of inoperable stage I non-small cell lung cancer. J Cancer Res Ther 13:683–688
Wei Z, Ye X, Yang X et al (2015) Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol 38:135–142
Wei Z, Ye X, Yang X et al (2015) Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol 32:464
Zhong L, Sun S, Shi J et al (2017) Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis 9:590–597
Ye X, Fan W, Chen JH et al (2015) Chinese expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer 6:112–121
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 273:241–260
Kong G, Anyarambhatla G, Petros WP et al (2000) Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 60:6950–6957
Goldberg SN, Saldinger PF, Gazelle GS et al (2001) Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology 220:420–427
Goldberg SN, Girnan GD, Lukyanov AN et al (2002) Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology 222:797–804
Goldberg SN, Kamel IR, Kruskal JB et al (2002) Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol 179:93–101
Solazzo SA, Ahmed M, Schor-Bardach R et al (2010) Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology 255:62–74
Ryan ER, Sofocleous CT, Schöder H et al (2013) Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology 268:288–295
Gomez DR, Blumenschein GR Jr, Lee JJ et al (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 17:1672–1682
Erinjeri JP, Thomas CT, Samoilia A et al (2013) Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol 24:1105–1112
Ni Y, Ye X, Wan C et al (2015) Percutaneous microwave ablation (MWA) increased the serum levels of VEGF and MMP-9 in stage I non-small cell lung cancer (NSCLC). Int J Hyperthermia. 1–5. https://doi.org/10.1080/02656736.2017.1284350
Sun YH, Song PY, Guo Y et al (2015) Effects of microwave ablation or its combination with whole-body chemotherapy on serum vascular endothelial growth factor levels in patients with stage IIIB/IV NSCLC. Genet Mol Res 14:1001
Schueller G, Kettenbach J, Sedivy R et al (2004) Heat shock protein expression induced by percutaneous radiofrequency ablation of hepatocellular carcinoma in vivo. Int J Oncol 24:609–613
Rai R, Richardson C, Flecknell P, Robertson H, Burt A, Manas DM (2005) Study of apoptosis and heat shock protein (HSP) expression in hepatocytes following radiofrequency ablation (RFA). J Surg Res 129:147–151
Guan HT, Wang J, Yang M, Song L, Tong XQ, Zou YH (2013) Changes in immunological function after treatment with transarterial chemoembolization plus radiofrequency ablation in hepatocellular carcinoma patients. Chin Med J (Engl) 126:3651–3655
Takaki H, Imai N, Thomas CT et al (2017) Changes in peripheral blood T-cell balance after percutaneous tumor ablation. Minim Invasive Ther Allied Technol 26(6):331–337
Stinchcombe TE, Socinski MA (2011) Maintenance therapy in advanced non-small cell lung cancer: current status and future implications. J Thorac Oncol 6:174–182
Novello S, Milella M, Tiseo M et al (2011) Maintenance therapy in NSCLC: why? To whom? Which agent? J Exp Clin Cancer Res 30:50
Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE (2008) Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247(3):871–879
Abbas G, Pennathur A, Landreneau RJ, Luketich JD (2009) Radiofrequency and microwave ablation of lung tumors. J Surg Oncol 100:645–650
Zheng A, Wang X, Yang X et al (2014) Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 98:243–248
Acknowledgments
The authors thank Professor Luigi Solbiati for medical writing assistance.
Funding
This study has received funding from the Funding Development Center for Medical Science and Technology National Health and Family Planning Commission of People’s Republic of China (W2015XR03), Shandong Province Medical and Health Science and Technology Development Projects (2014WS0346), and Technology Development Project the China international Medical Exchange Foundation (2012G0021824).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xin Ye.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Both Fan Li and Hua Fan have significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects patients in this study.
Ethical approval
Institutional Review Board approval was obtained from all centers.
Methodology
• prospective
• randomized controlled trial
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 411 kb)
Rights and permissions
About this article
Cite this article
Wei, Z., Yang, X., Ye, X. et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. Eur Radiol 30, 2692–2702 (2020). https://doi.org/10.1007/s00330-019-06613-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06613-x