Abstract
Purpose
To evaluate in vivo T2 mapping as quantitative, imaging-based biomarker for meniscal degeneration in humans, by studying the correlation between T2 relaxation time and degree of histological degeneration as reference standard.
Methods
In this prospective validation study, 13 menisci from seven patients with radiographic knee osteoarthritis (median age 67 years, three males) were included. Menisci were obtained during total knee replacement surgery. All patients underwent pre-operative magnetic resonance imaging using a 3-T MR scanner which included a T2 mapping pulse sequence with multiple echoes. Histological analysis of the collected menisci was performed using the Pauli score, involving surface integrity, cellularity, matrix organization, and staining intensity. Mean T2 relaxation times were calculated in meniscal regions of interest corresponding with the areas scored histologically, using a multi-slice multi-echo postprocessing algorithm. Correlation between T2 mapping and histology was assessed using a generalized least squares model fit by maximum likelihood.
Results
The mean T2 relaxation time was 22.4 ± 2.7 ms (range 18.5–27). The median histological score was 10, IQR 7–11 (range 4–13). A strong correlation between T2 relaxation time and histological score was found (rs = 0.84, CI 95% 0.64–0.93).
Conclusion
In vivo T2 mapping of the human meniscus correlates strongly with histological degeneration, suggesting that T2 mapping enables the detection and quantification of early compositional changes of the meniscus in knee OA.
Key Points
• Prospective histology-based study showed that in vivo T 2 mapping of the human meniscus correlates strongly with histological degeneration.
• Meniscal T 2 mapping allows detection and quantifying of compositional changes, without need for contrast or special MRI hardware.
• Meniscal T 2 mapping provides a biomarker for early OA, potentially allowing early treatment strategies and prevention of OA progression.
Similar content being viewed by others
Introduction
The pivotal role of the meniscus in knee osteoarthritis (OA) has attracted considerable attention among researchers for decades. Not only is meniscal damage a radiological sign of OA—up to 91% of the patients with symptomatic knee OA have coexisting meniscal tears [1]—a torn meniscus is also one of the strongest risk factors for the development and progression of knee OA [2,3,4,5]. Although the complex role of meniscal tissue composition in the etiology of meniscal tears and the subsequent development of knee OA is not entirely clear, it has become increasingly evident that the menisci play a critical role in the long-term health of the knee joint.
Hence, the ability to objectively assess meniscal tissue quality and composition is of key importance, particularly in patients at risk for developing knee OA [2]. In order to study the etiology of meniscal tears and meniscal degeneration in knee OA development and progression and to allow early interventions and prevention of progression, changes in meniscal tissue composition need to be detected before gross morphological changes occur.
Using conventional magnetic resonance (MR) imaging, measuring such changes in meniscal tissue composition prior to surface breakdown is challenging. Recent developments in quantitative MR imaging techniques, such as T2 mapping and T1rho, have made great progress in addressing this challenge [6, 7]. Among quantitative MR imaging techniques, T2 mapping is the most commonly used in knee OA research [8, 9]. Based on properties of biochemical tissue components, quantitative analysis of T2 relaxation times can reveal the composition of extracellular matrix, without the need for contrast or special MR hardware [6, 10]. Increased T2 relaxation times indicate damage to the collagen network and a decrease in water content, both signals of tissue degeneration [11].
Recent studies have shown the potential of T2 relaxation time as biomarker to quantify meniscal degeneration in patients with knee OA [6, 12,13,14], yet validation studies of meniscal T2 mapping are limited. Validation of T2 mapping using histological analysis (the gold standard for tissue changes) was performed in one previous study [7]. In that study, T2 mapping was performed ex vivo; however, it is unknown how well T2 measurements, obtained ex vivo, reflect the actual in vivo situation. To our knowledge, validation of in vivo meniscal T2 mapping, using histological analysis as reference test, has not been performed.
We aimed to validate in vivo meniscal T2 mapping in patients with knee OA by evaluating the correlation between T2 mapping and histological reference standards for meniscal degeneration.
Materials and methods
Study design and participants
Our prospective observational study was conducted between April 2016 and July 2017. Meniscal specimens were obtained from patients with primary end-stage knee OA undergoing elective total knee replacement surgery at our institution. Participants were selected consecutively. Study approval was granted by the institutional Medical Ethical Committee (MEC-2012-218), and written informed consent was obtained from all participants.
Assessment of radiological knee OA
The assessment of radiological knee OA is described in Appendix 1 in the Supplementary Material.
MR image acquisition
MR imaging was performed on a 3-Tesla (T) MR unit (Discovery MR750, GE Healthcare), 1 day prior to surgery. The MR imaging protocol included routine morphological knee sequences (proton density–weighted sequences in sagittal, coronal, and axial plane; T2-weighted sequences with fat saturation (Fat-Sat) in sagittal, coronal, and axial plane) and a sagittal 3D Fat-Sat fast spin-echo (FSE) T2 mapping sequence with multiple echoes. An 8-channel S&R rigid dedicated knee coil (GE Healthcare) was used. Sequence parameters are displayed in Table 1.
Harvesting of meniscal tissue and histological analysis
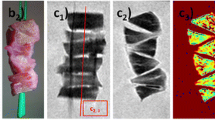
Meniscal specimens were obtained intraoperatively, during total knee replacement surgery. If present, both medial and lateral menisci were harvested; meniscal samples were stored in formaldehyde. Within 3 days of harvesting, menisci were cut in a standardized way according to Pauli et al [15] (Fig. 1). For each meniscus, the anterior horn and the posterior horn were processed. The menisci were cut at 45° (for the anterior horn) and 135° (for the posterior horn) angles relative to the sagittal plane (Fig. 1a). Meniscal samples were cut along two different planes: the vertical plane and the horizontal plane. The vertical section provided an overview of the longitudinally oriented collagen bundles and the tibial and femoral surfaces of the meniscus (Fig. 1c). The horizontal section, cut from the inner rim to the vascular zone at a 30° angle relative to the tibial plateau, revealed the parallel organization of the collagen bundles and matrix morphology (Fig. 1b).
Preparation of meniscal samples. Example of a grossly intact lateral meniscus harvested during total knee arthroplasty in the left knee of a 59-year-old female with medial compartment knee OA (Kellgren and Lawrence grade 4). a Cutting the meniscus according to the method of Pauli et al: vertical cut. b Horizontal cut, from the inner rim to the vascular zone at a 30° angle relative to the tibial plateau. c Detail view of the vertical cut of the posterior horn
The samples were fixed, dehydrated in alcohol, and infiltrated with paraffin. Next, meniscal samples were paraffin-embedded and sectioned using a microtome (MR2235, Leica-Biosystems) into 6-μm sections. To provide an overview of the overall tissue organization and to assess border integrity, cellularity, and cell morphology, sections were stained using hematoxylin and eosin. Safranin O-fast green and Alcian blue stains were used to evaluate proteoglycan content and mucoid degeneration, respectively. To assess collagen fiber organization, Picrosirius red stain was used. Stained sections were visualized using (polarized-) light microscopy (Olympus-BX50, Olympus-Optical) [16]. To assess the histological degree of degeneration, the validated, semi-quantitative Pauli score [15] was performed by two investigators with 4 years of experience in musculoskeletal research (Table 2). Both investigators were blinded to patient information and imaging outcomes. They examined all meniscal samples individually; in case of discrepancies, sections were assessed in consensus.
Quantitative MR image analysis
On T2 mapping images, meniscal regions of interest (ROIs) were manually segmented by a researcher with a medical degree and 4 years of experience in musculoskeletal research (Fig. 2), who was blinded to patient information and histology outcomes. Meniscal segmentation was performed using an image collected with the echo time (TE) showing optimal contrast between menisci and surrounding tissues (TE 7.3 ms).
Great care was taken to match MR imaging ROIs and histological ROIs. As described earlier, histological tissue processing was performed using predefined anatomical regions: the most central part of the anterior horn and the most central part of the posterior horn. As histological samples were cut in a fixed and standardized way, MR imaging ROIs were matched to histological ROIs. To do so, we identified the most central slice through the medial and lateral meniscus (defined as the sagittal slice depicting the maximum width of the anterior horn and posterior horn as individual triangles) along with the neighboring slices medially and laterally. Four ROIs were defined per patient: the anterior and posterior horn of the medial and lateral meniscus. All ROIs consisted of three consecutive slices: the most central slice along with the adjacent slice medially and laterally. MR imaging scout views, using T2-weighted images in the coronal and axial plane, were used to verify that the ROIs were correctly defined (i.e., that they matched histological ROIs).
For MR image postprocessing, in-house developed registration and fitting algorithms in MATLAB (R2011a; The MathWorks) were used [17]. Automated rigid registration in 3D was used for motion compensation [17]. Similar to previous studies [18, 19], we excluded all images with TE above 30 ms because of the very low signal-to-noise-ratio in meniscal tissue (Table 1). To reduce effects of possible outliers within ROIs, T2 relaxation times were weighted by the reciprocal of the uncertainty of the estimated T2 relaxation time in each voxel. This uncertainty was measured with the square root of the Cramer-Rao lower bound, which gives a lower bound for the standard deviation of the estimated T2 relaxation time [17]. The weighted T2 mapping relaxation times for each ROI were averaged over the three consecutive MR imaging slices, further referred to as mean T2 relaxation time [17].
Statistical analysis
Descriptive statistics for all available variables, including demographics, T2 relaxation times per meniscal ROI, and histological scores, are reported. Normality was tested using the Shapiro-Wilk tests. Normally distributed data were presented as mean with standard deviation; non-normally distributed data were presented as median with interquartile range (IQR).
Interobserver reliability of histological scoring was tested using two-way intraclass correlation coefficients (ICCs) of absolute agreement, taking single measurements.
We performed a linear mixed-effects model to assess the correlation between T2 relaxation times and histological scores, where T2 relaxation times were considered as dependent variable and histological score as independent variable. We employed the generalized least squares function in the “nlme” library in the statistical software “R” [20] allowing to calculate the correlation in repeated measures data (i.e., in datasets that include multiple measures per patient). Age, BMI, and sex were tested as potential covariates since they might impact T2 values. A backward variable selection and the likelihood ratio test were used for this purpose. Subgroup analyses were performed using a linear mixed-effects model, regarding regional differences.
Statistical analyses were performed using R version 3.4.2 (2017) [20].
Results
Patient characteristics
In total, 13 menisci were collected from 7 patients with knee OA: six medial and seven lateral menisci. There was a slight overall female predominance of 57%; the median age of patients was 67 years (range 59–74). None of the menisci showed a macroscopic tear. Patient characteristics are shown in Table 3.
Radiographic knee OA
Patients had either moderate radiographic knee OA (KLG 3, n = 3) or severe radiographic knee OA (KLG 4, n = 4).
T2 relaxation time in meniscal tissue
The mean meniscal T2 relaxation time was 22.4 ± 2.7 ms (range 18.5–27). In addition to overall mean T2 relaxation times (i.e., the mean of measurements from all ROIs), mean T2 relaxation times were calculated for the meniscal ROIs (medial anterior and posterior, lateral anterior and posterior) separately, reported in Table 4. Only ROIs of which the corresponding histological meniscal region was available were included in analyses. Highest T2 relaxation times were found in the medial anterior horn of the meniscus. Statistical significantly higher T2 relaxation times were found in the medial menisci than in the lateral menisci (p = 0.005). No statistically significant differences between the anterior and posterior meniscal horns in T2 relaxation time were found (p = 0.14). Representative T2 mapping findings are displayed in Fig. 3i, j.
Representative images of histological findings in meniscal tissue and corresponding T2 mapping images. a, c, e, g Posterior horn of lateral meniscus of a 67-year-old female with knee OA (Kellgren and Lawrence grade 3), with a mean T2 relaxation time of 18.6 ms and a histological score of 5. b, d, f, h Posterior horn of medial meniscus of a 66-year-old female with knee OA (Kellgren and Lawrence grade 4) with a mean T2 relaxation time of 26.9 ms and a histological score of 13. a, b Surface integrity (HE staining, × 10 zoom). c, d Cellularity (HE staining, × 40 zoom). e, f Collagen organization (Picrosirius red staining, × 5 zoom). g, h Collagen matrix staining intensity, a decreased intensity of green staining indicates disruption in collagen matrix (Saf O-green staining, × 10 zoom). i, j Corresponding non-contrast sagittal T2 mapping images with color map of the meniscus. The color bar on the right shows the range of T2 relaxation times
Histological findings in meniscal tissue
In two patients, all four meniscal regions (medial anterior, medial posterior, lateral anterior, and lateral posterior) could be harvested. In the remaining five patients, as a result of partial maceration of the menisci due to end-stage knee osteoarthritis or severe damage during surgery, not all four regions could be harvested (only three regions possible in four patients and a single region in one patient). In total, 21 out of 28 meniscal regions were used for histological analysis (medial anterior: n = 6, medial posterior: n = 5, lateral anterior: n = 5, lateral posterior: n = 5).
The interobserver reliability of histological scoring between the two observers was excellent (ICC 0.95, CI 95% 0.79–0.99). We found an overall median histological score of 10, IQR 7–11 (range 4–13). Mean histological scores per meniscal ROI are shown in Table 4. As for T2 relaxation times, the highest histological scores were found in the medial anterior horn of the meniscus and histological scores were found to be higher in the medial menisci than in the lateral menisci (p = 0.007). Also, no statistically significant differences between the anterior and posterior meniscal horns in histological score were found (p = 0.20). Representative histological findings are displayed in Fig. 3a–h.
Correlation between T2 mapping and histological scores
In the linear mixed-effects model, the variables age, sex, and BMI were not statistically significant and were excluded from the model. To incorporate the potential effect of repeated measures (i.e., multiple measures per patient), the model has been statistically adjusted. A strong correlation between T2 mapping and histology (correlation coefficient 0.85, CI 95% 0.68–0.93) was found (Fig. 4).
Discussion
In this study, we assessed the correlation between in vivo meniscal T2 mapping and histology in patients with radiographic knee OA. We demonstrated that meniscal T2 relaxation times in patients with knee OA show a strong correlation with the degree of histological degeneration. These findings indicate the potential of T2 relaxation times, obtained with in vivo T2 mapping, as non-invasive imaging biomarker for meniscal degeneration.
The results of our study are in line with those of previous research on meniscal T2 mapping where no histological analysis was performed. These studies showed that T2 mapping can differentiate between healthy patients and those with knee OA. Zarins et al found that meniscal T2 mapping discriminated between healthy and severe OA, but not between healthy and mild OA, and only in the posterior meniscal horns [19]. Rauscher and colleagues validated meniscal T2 mapping and T1rho against radiographical and clinical OA in subjects without OA, mild OA, and severe OA. They observed significant differences in T2 and T1rho values between subject groups and concluded that T2 mapping was more useful than T1rho for differentiating subject groups [13]. In addition to OA patients, T2 mapping has been investigated in patients with acute knee injury. Significantly higher T2 relaxation times were reported in patients with an anterior cruciate ligament rupture, compared with those in healthy controls [12]. To our knowledge, this is the first study to investigate the validity of in vivo meniscal T2 mapping in osteoarthritic patients, using histology as the reference test. Recently, Nebelung et al performed a comprehensive validation study of multiple quantitative MR imaging techniques, including T2 mapping, T1rho, and ultrashort echo time-enhanced T2* (UTE T2*) [7]. Histological analysis of meniscal samples from total knee replacement surgeries was used as the reference standard. In their study, strongest correlations between qMRI values and histology were found for T2 mapping and UTE T2*. In contrast to the present study, their T2 mapping measurements were performed ex vivo. Whether T2 measurements, obtained ex vivo, reflect the actual in vivo situation could be questioned. Several factors in ex vivo experiments may affect T2 relaxation times. First, storage of meniscal samples in medium and changes in tissue hydration may have potentially affected T2 measurements [7, 14]. Second, in ex vivo experiments, samples are typically scanned at room temperature and not at body temperature, potentially influencing T2 relaxation times. Last, ex vivo quantitative MR imaging experiments usually have different acquisition parameters, such as the number and duration of echo times, field of view, and acquisition matrix, compared with in vivo experiments [7, 21]. In addition to the differences between ex vivo and in vivo measurements, Nebelung and colleagues used a simplified, non-validated version of the Pauli score (the Williams score) to assess histological degeneration. These factors may have caused the lower correlation coefficient (r 0.65) between T2 mapping and histology in their study compared to ours.
T2 relaxation times have been increasingly used to assess meniscal tissue composition [7, 13, 14, 19], yet concerns have been raised that meniscal T2 mapping can be challenging due to the short T2 components and the heterogeneity of meniscal tissue [22,23,24]. In general, multi-echo T2 mapping sequences for knee OA have echo time (TE) values ranging from 10 to 100 ms [25], and mean T2 relaxation times of healthy menisci have been reported to be 11 ± 4 ms [13]. In previous studies, it has therefore been suggested that quantitative MR imaging techniques that obtain extremely short echo times, such as UTE T2*, may be more suitable to quantify menisci than standard spin-echo-based T2 mapping [23, 26, 27]. In the earlier mentioned study by Nebelung and colleagues, correlations between UTE T2* values and histology were comparable with correlations between T2 values and histology. However, they state that their choice of TE may not have been optimal for T2 mapping of the meniscus: acquired TE values ranged from 10 to 160 ms (TE 20–60 ms were used for analysis). Also, a 2D sequence was used for T2 mapping while a 3D sequence was used for UTE T2*, and single-slice quantitative analysis was performed, which may have influenced MRI outcomes and correlations [28]. In the present study, we took great care optimizing T2 mapping sequence parameters. We used a 3D spin-echo-based T2 mapping sequence with TE values ranging from 3 to 68 ms (TE 3–27 ms were used for analysis) and performed multi-slice quantitative analyses. We found a promising correlation between T2 values and histology (r 0.84, CI 95% 0.64–0.93), suggesting that in vivo spin-echo-based T2 mapping can provide accurate T2 measurements in menisci.
The results of the present study suggest that T2 relaxation times, obtained with in vivo T2 mapping, can potentially be used as non-invasive biomarker to detect early changes in meniscal tissue that indicate degeneration. T2 mapping allows a relatively wide range of TEs, with TE values short enough to assess menisci but long enough to assess articular cartilage [29,30,31]. Therefore, it may be a promising technique to detect early changes in various tissues involved in OA. The detection of early tissue changes, indicating degeneration, would allow a better understanding of the etiology and development of knee OA. Furthermore, it would allow the identification of patients at early OA stages, before irreversible damage occurs. Also, it would improve the monitoring of disease progression and treatment response. The long-term goal would be to allow the detection and monitoring of early tissue changes that indicate an increased risk for knee OA, potentially enabling early treatment strategies for knee OA.
This study has several limitations that should be considered. First, we had a limited sample size. Although a strong correlation was found between T2 values and histology, the small sample size may have impeded our statistical power. It should be noted, however, that meniscal degeneration was quite variable within the study population; the included menisci showed a relatively wide range of T2 values and histological score. Another limitation of the present study is that the meniscal body was not evaluated. The results of the present study may therefore only be generalizable for the meniscal horns. Also, we did not differentiate between meniscal zones (e.g., radially inner versus radially outer zone). The Pauli scoring system, which we used for histological grading of meniscal degeneration, does not distinguish meniscal zones. As the entire cross section needs to be assessed, a separate score for different meniscal zones is not possible. Finally, we could not include all meniscal regions of all menisci, as a result of complete maceration and/or severe damage during surgery. This issue should be considered for generalizing the results of this study. Future studies with greater sample size, and with further anatomical and zonal differentiation, should be conducted to reproduce our study results.
In conclusion, in vivo T2 mapping of the human meniscus provides accurate measurements of meniscal degeneration in patients with knee osteoarthritis. By quantifying subsurface meniscal changes, T2 mapping potentially provides a non-invasive imaging biomarker for meniscal degeneration.
Abbreviations
- BMI:
-
Body mass index
- CI 95%:
-
95% confidence interval
- Fat-Sat:
-
Fat saturation
- FSE:
-
Fast spin echo
- ICC:
-
Intraclass correlation coefficient
- IQR:
-
Interquartile range
- KLG:
-
Kellgren and Lawrence grade
- MR:
-
Magnetic resonance
- OA:
-
Osteoarthritis
- ROI:
-
Region of interest
- T:
-
Tesla
- TE:
-
Echo time
References
MacFarlane LA, Yang H, Collins JE et al (2017) Associations among meniscal damage, meniscal symptoms and knee pain severity. Osteoarthritis Cartilage 25:850–857
McDermott I (2011) Meniscal tears, repairs and replacement: their relevance to osteoarthritis of the knee. Br J Sports Med 45:292–297
Katz JN, Martin SD (2009) Meniscus--friend or foe: epidemiologic observations and surgical implications. Arthritis Rheum 60:633–635
Ding C, Martel-Pelletier J, Pelletier JP et al (2007) Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol 34:776–784
Antony B, Driban JB, Price LL et al (2017) The relationship between meniscal pathology and osteoarthritis depends on the type of meniscal damage visible on magnetic resonance images: data from the Osteoarthritis Initiative. Osteoarthr Cartil 25:76–84
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS (2013) Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartil 21:1474–1484
Nebelung S, Tingart M, Pufe T, Kuhl C, Jahr H, Truhn D (2016) Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skeletal Radiol 45:1649–1660
Guermazi A, Roemer FW, Burstein D, Hayashi D (2011) Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther 13:247
Welsch GH, Scheffler K, Mamisch TC et al (2009) Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn Reson Med 62:544–549
Hofmann FC, Neumann J, Heilmeier U et al (2018) Conservatively treated knee injury is associated with knee cartilage matrix degeneration measured with MRI-based T2 relaxation times: data from the osteoarthritis initiative. Skeletal Radiol 47:93–106
Arno S, Bell CP, Xia D et al (2016) Relationship between meniscal integrity and risk factors for cartilage degeneration. Knee 23:686–691
Wang A, Pedoia V, Su F et al (2016) MR T1ρ and T2 of meniscus after acute anterior cruciate ligament injuries. Osteoarthr Cartil 24:631–639
Rauscher I, Stahl R, Cheng J et al (2008) Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology 249:591–600
Son M, Goodman SB, Chen W, Hargreaves BA, Gold GE, Levenston ME (2013) Regional variation in T1ρ and T2 times in osteoarthritic human menisci: correlation with mechanical properties and matrix composition. Osteoarthr Cartil 21:796–805
Pauli C, Grogan SP, Patil S et al (2011) Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr Cartil 19:1132–1141
Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455
Bron EE, van Tiel J, Smit H et al (2013) Image registration improves human knee cartilage T1 mapping with delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Eur Radiol 23:246–252
Wang L, Chang G, Bencardino J et al (2014) T1rho MRI of menisci in patients with osteoarthritis at 3 Tesla: a preliminary study. J Magn Reson Imaging 40:588–595
Zarins ZA, Bolbos RI, Pialat JB et al (2010) Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthr Cartil 18:1408–1416
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/. Accessed 17 Aug 2018
van Tiel J, Kotek G, Reijman M et al (2016) Is T1ρ mapping an alternative to delayed gadolinium-enhanced MR imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology 279:523–531
Tsai PH, Chou MC, Lee HS et al (2009) MR T2 values of the knee menisci in the healthy young population: zonal and sex differences. Osteoarthr Cartil 17:988–994
McWalter EJ, Gold GE (2012) UTE T2* mapping detects sub-clinical meniscus degeneration. Osteoarthr Cartil 20:471–472
Ghadially FN, Lalonde JM, Wedge JH (1983) Ultrastructure of normal and torn menisci of the human knee joint. J Anat 136:773–791
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368
Sneag DB, Shah P, Koff MF, Lim WY, Rodeo SA, Potter HG (2015) Quantitative ultrashort echo time magnetic resonance imaging evaluation of postoperative menisci: a pilot study. HSS J 11:123–129
Williams A, Qian Y, Golla S, Chu CR (2012) UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthr Cartil 20:486–494
Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE (2015) T2 relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging 42:105–113
Peterfy CG, Schneider E, Nevitt M (2008) The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil 16:1433–1441
Oei EH, van Tiel J, Robinson WH, Gold GE (2014) Quantitative radiologic imaging techniques for articular cartilage composition: toward early diagnosis and development of disease-modifying therapeutics for osteoarthritis. Arthritis Care Res (Hoboken) 66:1129–1141
Li X, Cheng J, Lin K et al (2011) Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 29:324–334
Acknowledgements
We would like to thank Nicole Kops for technical assistance regarding histological experiments and Adam Weir for his help regarding scientific writing. In addition, the authors would like to thank the Department of Orthopedic Surgery of Erasmus MC University Medical Center for their cooperation in including patients and collecting meniscal tissue.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is EHG Oei.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: EHG Oei receives (non-financial) research support from GE Healthcare.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eijgenraam, S.M., Bovendeert, F.A.T., Verschueren, J. et al. T2 mapping of the meniscus is a biomarker for early osteoarthritis. Eur Radiol 29, 5664–5672 (2019). https://doi.org/10.1007/s00330-019-06091-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06091-1