Abstract
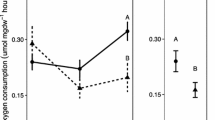
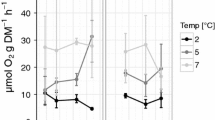
The waters of the Southern Ocean exhibit extreme seasonality in primary production, with marine life living below 0 °C for much of the year. The metabolic cold adaptation (MCA) hypothesis suggests that polar species need elevated basal metabolic rates to enable activity in such cold which should result in higher metabolic rates, or at least rates similar to temperate species. This study aimed to test whether any of the five common marine invertebrates around Adelaide Island (Western Antarctic Peninsula) displayed MCA: the suspension-feeding holothurian Heterocucumis steineni, the grazing limpet Nacella concinna, and the omnivorous brittle star, cushion star and sea-urchin Ophionotus victoriae, Odontaster validus and Sterechinus neumayeri, respectively. We also tested a second hypothesis that secondary consumers will exhibit less seasonal variation of metabolic rate than primary consumers. Routine oxygen consumption was measured in both the austral summer and winter using closed circuit respirometry techniques. Metabolic rates for all the species studied were low compared with temperate species, in a fashion consistent with expected temperature effects on biological systems and, therefore, the data do not support MCA. All the species studied showed significant seasonal differences for a standard mass animal except N. concinna. In two species N. concinna and H. steineni, size affected the seasonality of metabolism. There was no difference in seasonality of metabolism between primary and secondary consumers. Thus, for secondary consumers seasonal factors, most likely food availability and quality, vary enough to impact metabolic rates, and produce seasonal metabolic signals at all trophic levels. Other factors such as reproductive status that are linked to seasonal signals may also have contributed to the metabolic variation across trophic levels.





Similar content being viewed by others
References
Addo-Bediako A, Chown SL, Gaston KJ (2002) Metabolic cold adaptation in insects: a large-scale perspective. Funct Ecol 16:332–338
Arrigo KR, van Dijken G, Pabi S (2008) Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett 35:L19603
Barnes DKA (1995) Seasonal and annual growth in erect species of Antarctic bryozoans. J Exp Mar Biol Ecol 188:181–198
Barnes DKA, Clarke A (1995) Seasonality of feeding activity in Antarctic suspension feeders. Polar Biol 15:335–340
Belman BW, Giese A (1974) Oxygen consumption of an asteroid and an echinoid from the Antarctic. Biol Bull 146:157–164
Blackmer AL (2005) Exploring individual quality: basal metabolic rate and reproductive performance in storm-petrels. Behav Ecol 16:906–913
Bowden DA (2005) Seasonality of recruitment in Antarctic sessile marine benthos. Mar Ecol Prog Ser 297:101–118
Brockington S, Peck LS (2001) Seasonality of respiration and ammonium excretion in the Antarctic echinoid Sterechinus neumayeri. Mar Ecol Prog Ser 219:159–168
Brockington S, Clarke A, Chapman A (2001) Seasonality of feeding and nutritional status during the austral winter in the Antarctic sea urchin Sterechinus neumayeri. Mar Biol 139:127–138
Chapelle G, Peck LS (1999) Gigantism oxygen availablity. Nature 399:114–115
Chapelle G, Peck LS, Clarke A (1994) Effects of feeding and starvation on the metabolic rate of the necrophagous Antarctic amphipod Waldeckia obesa. J Exp Mar Biol Ecol 183:63–76
Clarke A (1988) Seasonality in the Antarctic marine environment. Comp Biochem Physiol B 90:461–473
Clarke A (1993) Seasonal acclimatization and latitudinal compensation in metabolism: Do They Exist? Funct Ecol 7:139–149
Clarke A, Brockington S (2001) The relative influence of temperature and food on the metabolism of a marine invertebrate. J Exp Mar Biol Ecol 258:87–99
Clarke A, Fraser KP (2004) Why does metabolism scale with temperature? Funct Ecol 18:243–251
Clarke A, Johnston N (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Clarke A, Peck LS (1991) Physiology of polar marine zooplankton. Polar Res 10:355–369
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Proc R Soc Lond B Biol Sci 362:149–166
Clarke A, Meredith MP, Wallace MI, Brandon MA, Thomas DN (2008) Seasonal and interannual variability in temperature, chlorophyll and macronutrients in northern Marguerite Bay, Antarctica. Deep-Sea Res (2 TOP Stud Oceanogr) 55:1988–2006
Fang J, Zhang J, Liu Y, Jiang Z, Mao Y, Fang J (2014) Effects of temperature and salinity on mortality and metabolism of Ophiopholis mirabilis. Mar Biol Res 11:157–167
Fraser KPP, Clarke A, Peck LS (2002) Feast and Famine in Antarctica: seasonal physiology in the limpet Nacella concinna. Mar Ecol Prog Ser 242:169–177
Fraser KPP, Peck LS, Clarke A (2004) Protein Synthesis, RNA Concentrations, Nitrogen Excretion, and Metabolism vary Seasonally in the Antarctic Holothurian Heterocucumis steineni. Physiol Biochem Zool 77:556–569
Fraser KP, Clarke A, Peck LS (2007) Growth in the slow lane: protein metabolism in the Antarctic limpet Nacella concinna (Strebel 1908). J Exp Biol 210:2691–2699
Gaitan-Espitia JD, Nespolo R (2014) Is there metabolic cold adaptation in terrestrial ectotherms? Exploring latitudinal compensation in the invasive snail Cornu aspersum. J Exp Biol 217:2261–2267
Grange LJ, Tyler PA, Peck LS, Cornelius N (2004) Long Term interannual cycles of the gametogenic ecology of the Antarctic brittle star Ophionotus victoriae. Mar Ecol Prog Ser 278:141–155
Grange LJ, Tyler PA, Peck LS (2007) Multi-year observations on the gametogenic ecology of the Antarctic seastar Odontaster validus. Mar Biol 153:15–23
Groeneveld J, Johst K, Kawaguchi S, Meyer B, Teschke M, Grimm V (2015) How biological clocks and changing environmental conditions determine local population growth and species distribution in Antarctic krill (Euphausia superba): a conceptual model. Ecol Model 303:78–86
Gyllenberg G, Lundqvist G (1979) The effects of temperature and salinity on the oxygen consumption of Eurytemora hirundoides (Crustacea, Copepoda). Ann Zool 16:205–208
Houlihan DF, Allan D (1982) Oxygen consumption of some Antarctic and British gastropods: An evaluation of cold adaptation. Comp Biochem Physiol A 73:383–387
Janecki T, Rakusa-Suszczewski S (2006) Biology and metabolism of Glyptonotus antarcticus (Eights) (Crustacea: Isopoda) from Admiralty Bay, King George Island, Antarctica. Polar Bioscience 19:29–42
Kock KH, Everson I (1998) Age, growth and maximum size of Antarctic Notothenoid Fish—revisited. In: Fishes of Antarctica. A biological overview. Springer
Krogh A (1916) The respiratory exchange of animals and man. Green and Co L, Longmans
Lardies MA, Bacigalupe LD, Bozinovic F (2004) Testing the metabolic cold adaptation hypothesis: an intraspecific latitudinal comparison. Evol Ecol 6:567–578
Luxmoore RA (1984) A comparison of the respiration rate of some Antarctic isopods with species from lower latitudes. Brit Antarct Surv B 62:53–65
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10:193–213
Morley SA, Peck LS, Miller AJ, Portner HO (2007) Hypoxia tolerance associated with activity reduction is a key adaptation for Laternula elliptica seasonal energetics. Oecologia 153:29–36
Morley SA, Berman J, Barnes DKA, De Juan Carbonell C, Downey RV, Peck LS (2016) Extreme phenotypic plasticity in metabolic physiology of Antarctic demosponges. Front Ecol Evol 3:157
Norrbin MF (1991) Gonad maturation as an indication of seasonal cycles for several species of small copepods in the Barents Sea. Polar Res 10:421–432
Obermüller BE, Morley SA, Barnes DKA, Peck LS (2010) Seasonal physiology and ecology of Antarctic marine benthic predators and scavengers. Mar Ecol Prog Ser 415:109–126
Obermüller BE, Morley SA, Clark MS, Barnes DKA, Peck LS (2011) Antarctic intertidal limpet ecophysiology: a winter-summer comparison. J Exp Mar Biol Ecol 403:39–45
Pearse JS, McClintock JB, Bosch I (1991) Reproduction of Antarctic benthic marine invertebrates: tempos, modes, and timing. Am Zool 31:65–80
Peck LS (2016) A cold limit to adaptation in the sea. Trends Ecol Evol 31:13–26
Peck LS, Barnes DKA (2004) Metabolic flexibility: the key to long-term evolutionary success in Bryozoa? Proc R Soc Lond B Biol Sci 271:18–21
Peck LS, Conway LZ (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. Geo Soc Spec Publ 177:441–450
Peck LS, Uglow RF (1990) Two methods for the assessment of the oxygen content of small volumes of seawater. J Exp Mar Biol Ecol 141:53–62
Peck LS, Brockington S, Brey T (1997) Growth and metabolism in the Antarctic brachiopod Liothyrella uva. Proc R Soc Lond B Biol Sci 352:851–858
Peck LS, Colman JG, Murray AWA (2000) Growth and tissue mass cycles in the infuanal bivalve Yoldia eightsi at Signy Island, Antarctica. Polar Biol 23:420–428
Peck LS, Heiser S, Clark MS (2016) Very slow embryonic and larval development in the Antarctic limpet Nacella polaris. Polar Biol 39:2273–2280
Rakusa-Suszczewski S (1982) The biology and metabolism of Orchomene plebs (Hurley 1965) (Amphipoda: Gammaridea) from McMurdo Sound, Ross Sea, Antarctic. Polar Biol 1:47–54
Ralph R, Maxwell GH (1977a) The oxygen consumption of the Antarctic lamellibranch Gaimardia trapesina in relation to cold adaptation in polar invertebrates. Brit Antarctic Surv B 45:41–46
Ralph R, Maxwell GH (1977b) The oxygen consumption of the Antarctic limpet Nacella concinna. Brit Antarctic Surv B 45:19–23
Román-González A, Scourse JDB, Paul G, Reynolds DJ, Richardson CA, Peck LS, Brey T, Hall IR (2017) Analysis of ontogenetic growth trends in two marine Antarctic bivalves Yoldia eightsi and Laternula elliptica: Implications for sclerochronology. Palaeontogr Palaeocl 465:300–306
Schaefer J, Walters A (2010) Metabolic cold adaptation and developmental plasticity in metabolic rates among species in the Fundulus notatus species complex. Funct Ecol 24:1087–1094
Seebacher F, White CR, Franklin CE (2014) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Chang 5:61–66
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Proc R Soc Lond B Biol Sci 362:2061–2078
Seibel BA, Oschlies A, Childress JJ (2012) The real limits to marine life: a further critique of the respiration index. Biogeosci Discuss 9:16521–16532
Stanwell-Smith D, Clarke A (1998) Seasonality of reproduction in the cushion star Odontaster validus at Signy Island, Antarctica. Mar Biol 131:479–487
Stanwell-Smith D, Peck LS, Clarke A, Murray AWA, Todd CD (1999) The distribution, abundance and seasonality of pelagic marine invertebrate larvae in the maritime Antarctic. Proc R Soc Lond B Biol Sci 354:471–484
Steffensen JF (2002) Metabolic cold adaptation of polar fish based on measurements of aerobic oxygen consumption: fact or artefact? Artefact! Comp Biochem Physiol A 132:789–795
Thyrring J, Rysgaard S, Blicher ME, Sejr MK (2015) Metabolic cold adaptation and aerobic performance of blue mussels (Mytilus edulis) along a temperature gradient into the High Arctic region. Mar Biol 162:235–243
Torre L, Servetto N, Eory ML, Momo F, Tatian M, Abele D, Sahade R (2012) Respiratory responses of three Antarctic ascidians and a sea pen to increased sediment concentrations. Polar Biol 35:1743–1748
Uliano E, Chaurasia A, Berna L, Agnisola C, D’Onofrio G (2010) Metabolic rate and genomic GC: what we can learn from teleost fish. Mar Genomics 3:29–34
Venables HJ, Clarke A, Meredith MP (2013) Wintertime controls on summer stratification and productivity at the western Antarctic Peninsula. Limnol Oceanogr 58:1035–1047
Watson S-A, Morley SA, Bates AE, Clark MS, Day RW, Lamare M, Martin SM, Southgate PC, Tan KS, Tyler PA, Peck LS (2013) Low global sensitivity of metabolic rate to temperature in calcified marine invertebrates. Oecologia 174:45–54
Wheeling RJ, Verde EA, Nestler JR (2007) Diel cycles of activity, metabolism, and ammonium concentration in tropical holothurians. Mar Biol 152:297–305
White CR, Alton LA, Frappell PB (2011) Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc R Soc Lond B Biol Sci 279:1740–1747
Whitney NM, Lear KO, Gaskins LC, Gleiss AC (2016) The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre). J Exp Mar Biol Ecol 477:40–46
Wohlschlag DE (1964) Respiratory metabolism and ecological characteristics of some fishes in McMurdo sound, Antarctica. In: biology of the Antarctic Seas. Antarctic Research series, vol 1. American Geophysics Union, pp 33–62
Acknowledgements
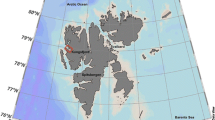
The authors would like to thank all members of the Rothera Research Station dive, boating and support teams from 2014 to 2016 for their help with collecting and maintaining animals. BAS diving is supported by the NERC National Facility for Scientific Diving at Oban. This project was funded by the Natural Environment Research Council. The authors confirm that sampling of all species and use of animals in experiments was in accordance with relevant guidelines and permits. Terri A Souster would particularly like to thank Jonathan Yates for help with animal sorting and in general for all his support and patience. Thanks to Melody Clark for critical reading of the manuscript. Thanks also to MAGIC for production of the map used in Fig. 1. The author would also like to acknowledge the anonymous referees who provided comments that led to a much improved manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
To the authors knowledge there is no conflict of interest in the production of this manuscript.
Rights and permissions
About this article
Cite this article
Souster, T.A., Morley, S.A. & Peck, L.S. Seasonality of oxygen consumption in five common Antarctic benthic marine invertebrates. Polar Biol 41, 897–908 (2018). https://doi.org/10.1007/s00300-018-2251-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2251-3