Abstract
Key message
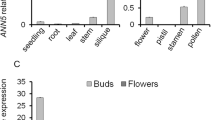
Two Arabidopsis ABC transporters, ABCG1 and ABCG16, are expressed in the tapetal layer, specifically after postmeiotic microspore release, and play important roles in pollen surface development.
Abstract
The male gametophytic cells of terrestrial plants, the pollen grains, travel far before fertilization, and thus require strong protective layers, which take the form of a pollen coat and a pollen wall. The protective surface structures are generated by the tapetum, the tissue surrounding the developing gametophytes. Many ABC transporters, including Arabidopsis thaliana ABCG1 and ABCG16, have been shown to play essential roles in the development of such protective layers. However, the details of the mechanism of their function remain to be clarified. In this study, we show that ABCG1 and ABCG16 are localized at the plasma membrane of tapetal cells, specifically after postmeiotic microspore release, and play critical roles in the postmeiotic stages of male gametophyte development. Consistent with this stage-specific expression, the abcg1 abcg16 double knockout mutant exhibited defects in pollen development after postmeiotic microspore release; their microspores lacked intact nexine and intine layers, exhibited defects in pollen mitosis I, displayed ectopic deposits of arabinogalactan proteins, failed to complete cytokinesis, and lacked sperm cells. Interestingly, the double mutant exhibited abnormalities in the internal structures of tapetal cells, too; the storage organelles of tapetal cells, tapetosomes and elaioplasts, were morphologically altered. Thus, this work reveals that the lack of ABCG1 and ABCG16 at the tapetal cell membrane causes a broad range of defects in pollen, as well as in tapetal cells themselves. Furthermore, these results suggest that normal pollen surface development is necessary for normal development of the pollen cytoplasm.





Similar content being viewed by others
Abbreviations
- ABC transporter:
-
ATP-binding cassette transporter
- AMS:
-
Aborted microspores
- TEK:
-
Transposable element silencing via AT-hook
- AGP:
-
Arabinogalactan protein
- TEM:
-
Transmission electron microscopy
References
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Chaves I, Regalado AP, Chen M, Ricardo CP, Showalter AM (2002) Programmed cell death induced by (β-d-galactosyl) 3 Yariv reagent in Nicotiana tabacum BY-2 suspension-cultured cells. Physiol Plant 116:548–553
Choi H, Jin JY, Choi S, Hwang JU, Kim YY, Suh MC, Lee Y (2011) An ABCG/WBC-type ABC transporter is essential for transport of sporopollenin precursors for exine formation in developing pollen. Plant J 65:181–193
Choi H, Ohyama K, Kim YY, Jin JY, Lee SB, Yamaoka Y, Muranaka T, Suh MC, Fujioka S, Lee Y (2014) The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat. Plant Cell Online 26:310–324
Coimbra S, Almeida J, Junqueira V, Costa ML, Pereira LG (2007) Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J Exp Bot 58:4027–4035
Dou XY, Yang KZ, Zhang Y, Wang W, Liu XL, Chen LQ, Zhang XQ, Ye D (2011) WBC27, an adenosine tri-phosphate-binding cassette protein, controls pollen wall formation and patterning in Arabidopsis. J Integr Plant Biol 53:74–88
Gadjev I, Stone JM, Gechev TS (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mole Biol 270:87–144
Gao M, Showalter AM (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J Cell Mole Biol 19:321–331
Heslop-Harrison J (1968) Pollen wall development. Science 161:230–237
Hsieh K, Huang AH (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell Online 19:582–596
Jia QS, Zhu J, Xu XF, Lou Y, Zhang ZL, Zhang ZP, Yang ZN (2015) Arabidopsis AT-hook protein TEK positively regulates the expression of arabinogalactan proteins for nexine formation. Mol Plant 8:251–260
Kang B (2010) Electron microscopy and high-pressure freezing of Arabidopsis. Methods Cell Biol 96:259–283
Karahara I, Kang BH (2014) High-pressure freezing and low-temperature processing of plant tissue samples for electron microscopy. Methods Mol Biol 1080:147–157
Lou Y, Xu XF, Zhu J, Gu JN, Blackmore S, Yang ZN (2014) The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nature Commun 5:3855
Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125:3789–3799
Piffanelli P, Ross JH, Murphy D (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Quilichini TD, Friedmann MC, Samuels AL, Douglas CJ (2010) ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol 154:678–690
Quilichini TD, Douglas CJ, Samuels AL (2014a) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot 114:1189–1201
Quilichini TD, Samuels AL, Douglas CJ (2014b) ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell 26:4483–4498
Reape TJ, Molony EM, McCabe PF (2008) Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59:435–444
Sanders PM, Bui AQ, Weterings K, McIntire K, Hsu YC, Lee PY, Truong MT, Beals T, Goldberg R (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Schindler T, Bergfeld R, Schopfer P (1995) Arabinogalactan proteins in maize coleoptiles: developmental relationship to cell death during xylem differentiation but not to extension growth. Plant J Cell Mole Biol 7:25–36
Scott R (1994) Pollen exine-the sporopollenin enigma and the physics of pattern. In: SEMINAR SERIES-SOCIETY FOR EXPERIMENTAL BIOLOGY. Cambridge University Press, pp 49–49
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell Online 2:755–767
Sorensen AM, Kröber S, Unte US, Huijser P, Dekker K, Saedler H (2003) The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J 33:413–423
Ting JT, Wu SS, Ratnayake C, Huang AH (1998) Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J Cell Mole Biol 16:541–551
Wallace S, Fleming A, Wellman CH, Beerling DJ (2011) Evolutionary development of the plant spore and pollen wall. AoB plants 2011:plr027
Wellman CH (2004) Origin, function and development of the spore wall in early land plants. Evolut Plant Physiol 21
Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA (2010) The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell Online 22:91–107
Xu J, Ding Z, Vizcay-Barrena G, Shi J, Liang W, Yuan Z, Werck-Reichhart D, Schreiber L, Wilson ZA, Zhang D (2014) ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell Online 26:1544–1556
Yadav V, Molina I, Ranathunge K, Castillo IQ, Rothstein SJ, Reed JW (2014) ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 26:3569–3588
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Ministry of Science, Information and Communication Technology, and Future Planning, Korea awarded to Y.L. (NRF-2015R1A2A1A01004294), Grants from the National Key Basic Research Developments Program, Ministry of Science and Technology, China (2013CB126902); National Transgenic Major Program (2016ZX08009003-003-007) awarded to D. Zhang, and by the Direct Grant for Research from the Chinese University of Hong Kong (4053089 and 3132797) to B. Kang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Y.-Il Park.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yim, S., Khare, D., Kang, J. et al. Postmeiotic development of pollen surface layers requires two Arabidopsis ABCG-type transporters. Plant Cell Rep 35, 1863–1873 (2016). https://doi.org/10.1007/s00299-016-2001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2001-3