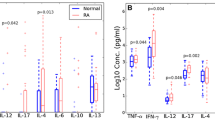
Abstract
We examined the functional activity of peripheral blood neutrophils and the complement system activation status in patients with rheumatoid arthritis (RA) undergoing infliximab/methotrexate combined therapy. We studied female RA patients under treatment with infliximab (3–5 mg/kg) and methotrexate (15–25 mg/week) who presented inactive (i-RA; n = 34, DAS-28 ≤ 2.6) or at least moderately active disease (a-RA; n = 29, DAS-28 > 3.2), and age-matched healthy women (n = 38). We measured the levels of reactive oxygen species (ROS) generation (chemiluminescence assay) and membrane expression of FcγRIIa/CD32, FcγRIIIb/CD16, CR1/CD35, and CR3/CD11b receptors (ELISA assay) in neutrophils. We also determined the hemolytic activity of the alternative and classical pathways of the complement system (spectrophotometry), serum levels of C5a and Bb (ELISA assay), and serum chemotactic activity (Boyden chamber). Compared with the control group, i-RA and a-RA patients exhibited: (1) increased neutrophil ROS production and membrane expression of FcγRIIa/CD32, FcγRIIIb/CD16, and CR1/CD35, indicating neutrophil activation; and (2) increased serum chemotactic activity and decreased activity of the alternative complement pathway, indicating systemic complement system activation. The levels of C-reactive protein in a-RA patients were augmented, compared with i-RA patients. Although infliximab/methotrexate combined therapy induced disease remission according to the DAS-28 criteria, both i-RA and a-RA patients still exhibited significant levels of systemic activation of neutrophils and the complement system.




Similar content being viewed by others
Abbreviations
- Anti-CCP:
-
Anti-cyclic citrullinated peptide antibody
- CHS:
-
Serum from healthy control subject
- CL:
-
Chemiluminescence
- CR:
-
Complement receptor
- CRP:
-
C-reactive protein
- DAS-28:
-
Disease activity score of 28 joints
- DMARD:
-
Disease-modifying anti-rheumatic drug
- EULAR:
-
European League against Rheumatism
- FcγR:
-
Fcγ receptors
- FITC:
-
Fluorescein isothiocyanate
- HA:
-
Hemolytic activity
- IC:
-
Immune complex
- IFM:
-
Infliximab
- MFI:
-
Median fluorescence intensity
- MTX:
-
Methotrexate
- PE:
-
Phycoerythrin
- RA:
-
Rheumatoid arthritis
- a-RA:
-
Active rheumatoid arthritis
- i-RA:
-
Inactive rheumatoid arthritis
- RAHS:
-
Serum from patient with rheumatoid arthritis
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-α
References
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
McInnes IB, Buckley CD, Isaacs JD (2016) Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol 12:63–68
Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V (2016) Human neutrophils in auto-immunity. Semin Immunol 28:159–173
American College of Rheumatology (2002) Guidelines for the management of rheumatoid arthritis—2002 update. Arthritis Rheum 46:328–346
Curtis JR, Singh JA (2011) The use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 33:679–707
Jani M, Barton A, Warren RB, Griffiths CEM, Chinoy H (2014) The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology 53:213–222
Dimopoulou D (2015) Infliximab as a treatment option for patients with rheumatoid arthritis and primary biliary cirrhosis. Rheumatol Int 35:1913–1916
Costa JO, Lemos LLP, Machado MAA, Almeida AM, Kakehasi AM, Araújo VE et al (2015) Infliximab, methotrexate and their combination for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Rev Bras Reumatol 55:146–158
Tanaka Y (2016) Stopping tumour necrosis factor-targeted biological DMARDs in rheumatoid arthritis. Rheumatology 55(suppl 2):ii15–ii22
Mota LM, Cruz BA, Albuquerque CP, Gonçalves DP, Laurindo IM, Pereira IA et al (2015) Update on the 2012 Brazilian Society of Rheumatology Guidelines for the treatment of rheumatoid arthritis: position on the use of tofacitinib. Rev Bras Reumatol 55:512–521
Pomirleanu C, Ancuta C, Miu S, Chirieac R (2013) A predictive model for remission and low disease activity in patients with established rheumatoid arthritis receiving TNF blockers. Clin Rheumatol 32:665–670
Shaffu S, Edwards J, Neame R, Hassan W (2013) Self-assessment of 28-joint disease activity scores by patients with rheumatoid arthritis on anti-TNF therapy. Rheumatology 52:576–578
Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE (2010) Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 9:531–535
Asquith DL, Miller AM, McInnes IB, Liew FY (2009) Animal models of rheumatoid arthritis. Eur J Immunol 39:2040–2044
Gierut A, Perlman H, Pope RM (2010) Innate immunity and rheumatoid arthritis. Rheum Dis Clin N Am 36:271–296
Chen M, Daha MR, Kallenberg CG (2010) The complement system in systemic autoimmune disease. J Autoimmun 34:276–286
Ballanti E, Perricone C, Greco E, Ballanti M, Di Muzio G, Chimenti MS et al (2013) Complement and autoimmunity. Immunol Res 56:477–491
Paoliello-Paschoalato AB, Marchi LF, Andrade MF, Kabeya LM, Donadi EA, Lucisano-Valim YM (2015) Fcγ and complement receptors and complement proteins in neutrophil activation in rheumatoid arthritis: contribution to pathogenesis and progression and modulation by natural products. Evid Based Complement Alternat Med 2015:429878
Paoliello-Paschoalato AB, Moreira MR, Azzolini AECS, Cavenaghi AA, Marzocchi-Machado CM, Donadi EA et al (2011) Activation of complement alternative pathway in rheumatoid arthritis: implications in peripheral neutrophils functions. Open Autoimmun J 3:1–9
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Frie JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
van Gestel AM, Haagsma CJ, van Riel PL (1998) Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 41:1845–1850
Paoliello-Paschoalato AB, Azzolini AECS, Cruz MFC, Marchi LF, Kabeya LM, Donadi EA et al (2014) Isolation of healthy individuals’ and rheumatoid arthritis patients’ peripheral blood neutrophils by the gelatin and Ficoll-Hypaque methods: comparative efficiency and impact on the neutrophil oxidative metabolism and Fcγ receptor expression. J Immunol Methods 412:70–77
Dougados M, Aletaha D, van Riel P (2007) Disease activity measures for rheumatoid arthritis. Clin Exp Rheumatol 25(5 Suppl 46):S22–S29
Oliveira RD, Junta CM, Oliveira FR, Silva LM, Donadi EA, Louzada-Junior P (2008) Share epitope, citrullinated cyclic peptide antibodies and smoking in Brazilian rheumatoid arthritis patients. Clinic Rev Allerg Immunol 34:32–35
Marzocchi-Machado CM, Alves CMOS, Azzolini AECS, Polizello ACM, Carvalho IF, Lucisano-Valim YM (2002) Fcγ and complement receptors: expression, role and cooperation to mediate the oxidative burst and degranulation of neutrophil of Brazilian SLE patients. Lupus 11:240–248
Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466
Zigmond SH, Hirsch JG (1973) Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med 137:387–410
Landi-Librandi AP, Chrysóstomo TN, Azzolini AECS, Recchia CGV, Uyemura SA, Assis-Pandochi AI (2007) Effect of the extract of the tamarind (Tamarindus indica) fruit on the complement system: studies in vitro and in hamsters submitted to a cholesterol-enriched diet. Food Chem Toxicol 45:1487–1495
Aletaha D, Blüml S (2016) Therapeutic implications of autoantibodies in rheumatoid arthritis. RMD Open 2:e000009
Kundu S, Ghosh P, Datta S, Ghosh A, Chattopadhyay S, Chatterjee M (2012) Oxidative stress as a potential biomarker for determining disease activity in patients with rheumatoid arthritis. Free Radic Res 46:1482–1489
den Broeder AA, Wanten GJ, Oyen WJ, Naber T, van Riel PL, Barrera P (2003) Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti-tumor necrosis factor-alpha monoclonal antibody in patients with rheumatoid arthritis. J Rheumatol 30:232–237
Lorenz HM, Antoni C, Valerius T, Repp R, Grünke M, Schwerdtner N et al (1996) In vivo blockade of TNF-α by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol 156:1646–1653
Wijngaarden S, van de Winkel JG, Bijlsma JW, Lafeber FP, van Roon JA (2008) Treatment of rheumatoid arthritis patients with anti-TNF-γ monoclonal antibody is accompanied by down-regulation of the activating Fcγ receptor I on monocytes. Clin Exp Rheumatol 26:89–95
Belostocki K, Pricop L, Redecha PB, Aydin A, Leff L, Harrison MJ et al (2008) Infliximab treatment shifts the balance between stimulatory and inhibitory Fcγ receptor type II isoforms on neutrophils in patients with rheumatoid arthritis. Arthritis Rheum 58:384–388
Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, Judex M et al (2002) Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum 46:934–945
Happonen KE, Heinegard D, Saxne T, Blom AM (2012) Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology 217:1088–1096
Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB (1995) Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med 1:237–243
Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR et al (2009) Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum 60:1923–1931
Volanakis JE (1982) Complement activation by C-reactive protein complexes. Ann N Y Acad Sci 389:235–250
Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE (2001) Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis Rheum 44:997–1002
Familian A, Voskuyl AE, van Mierlo GJ, Heijst HA, Twisk JW, Dijkmans BA et al (2005) Infliximab treatment reduces complement activation in patients with rheumatoid arthritis. Ann Rheum Dis 64:1003–1008
Wolbink GJ, Brouwer MC, Buysmann S, ten Berge IJ, Hack CE (1996) CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J Immunol 157:473–479
Elliot MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katasikis P et al (2008) Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum 58(Suppl 2):S92–S101
Brodeur JP, Ruddy S, Schwartz LB, Moxley G (1991) Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum 34:1531–1537
Di Franco M, Lucchino B, Spaziante M, Iannuccelli C, Valesini G, Iaiani G (2017) Lung infections in systemic rheumatic disease: focus on opportunistic infections. Int J Mol Sci 18:293
Alves CMOS, Marzocchi-Machado CM, Louzada-Junior P, Azzolini AECS, Polizello ACM, Carvalho IF, Lucisano-Valim YM (2008) Superoxide anion production by neutrophils is associated with prevalent clinical manifestations in systemic lupus erythematosus. Clin Rheumatol 27:701–708
Acknowledgements
The authors are grateful to Dr. Ana Paula Landi Librandi, from the School of Pharmaceutical Sciences of Ribeirão Preto (Ribeirão Preto, SP, Brazil) for helping us to perform the ELISA assays, and to Mrs. Patricia Vianna Bonini Palma and Mrs. Camila Cristina O. M. Bonaldo from the Hemotherapy Center of Ribeirão Preto (FUNDHERP, Ribeirão Preto, SP, Brazil) for their helpful technical assistance in the flow cytometry analyses.
Funding
The Brazilian funding agencies São Paulo Research Foundation (FAPESP; Grants 2013/22331-5, and 2013/20810-7), the National Council for Scientific and Technological Development (CNPq; Grants 151665/2010-9, 308111/2013/3), and the Coordination for the Improvement of Higher Education Personnel (CAPES) provided the fellowships and financial support.
Author information
Authors and Affiliations
Contributions
LFM and ABPP devised the project, designed the study, analyzed and interpreted the results, and drafted the manuscript. LFM conducted all the experiments. RDRO followed-up and selected the patients, and determined their clinical parameters. AECSA assisted in all the experiments, and in data processing and analysis. LMK assisted in statistical data analyses, and drafted and proofread the manuscript. EAD selected the patients and supervised the study. YMLV devised the project, searched for funding, and supervised the study. All authors discussed the results, and revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Research Ethics Committee of the Ribeirão Preto Medical School Hospital of the University of São Paulo (HC-FMRP-USP, Ribeirão Preto, SP, Brazil) approved the study protocol (HCRP n. 10097/2002). All the procedures conducted in humans complied with Resolution 466/12 of the Brazilian National Health Council, which follows the recommendations of the 1964 Helsinki declaration and its later amendments.
Informed consent
All the patients and healthy subjects signed the informed consent form to participate in this study.
Additional information
This study is part of the post-doctoral research project developed by Dr. Larissa Fávaro Marchi under supervision of Dr. Yara Maria Lucisano-Valim, at School of Pharmaceutical Sciences of Ribeirão Preto. The present institutional address of Dr. Marchi is: Faculdades Integradas Padre Albino, FIPA, Rua dos Estudantes 225, Catanduva, SP, 15809-144 Brazil.
Rights and permissions
About this article
Cite this article
Marchi, L.F., Paoliello-Paschoalato, A.B., Oliveira, R.D.R. et al. Activation status of peripheral blood neutrophils and the complement system in adult rheumatoid arthritis patients undergoing combined therapy with infliximab and methotrexate. Rheumatol Int 38, 1043–1052 (2018). https://doi.org/10.1007/s00296-018-3997-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-3997-1