Abstract
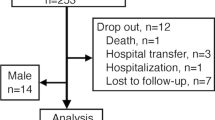
The objective of our prospective study is to specify the variability of densitometric response to Denosumab, given in the second line, and to try to understand the reasons. All menopausal patients with primary osteoporosis, treated by Denosumab in our centre from 2014 to 2015, were included in this open prospective work. At T0, the patient’s age, type of fracture, and previous treatments were collated. At T0 and T1, after 1 year of treatment by Dmab, a DXA of the spine and the hip and a determination of CTX were performed. Sixty-three patients aged 68.8 ± 8.3 years were included. The median number of treatments prescribed for osteoporosis before switch to Denosumab was 2.4. The median duration of these treatments was 7.2 years. At T1, CTX was less than 33 pg/ml (minimum threshold for our assay kit) in all patients. The median BMD in the spine increased by + 5.44% compared to T0. 14 patients in the upper quartile had a median BMD gain in the spine of + 11.07%. Fourteen patients in the lower quartile had a median BMD gain in the spine of + 0.6%. Only the duration of previous treatments, which was greater in the non-responder group, differed between these two groups. In the total cohort, the spinal densitometric gain was negatively correlated with the age of the patient at baseline (p = 0.04), the duration of previous treatment (p = 0.02), and positively with the CTX level (p = 0.05). The Dmab densitometric response is highly variable, partly explained by the duration of previous treatments and the level of bone resorption at initiation of treatment.

Similar content being viewed by others
References
McClung MR et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Lewiecki EM et al (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22:1832–1841
Cummings SR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Miller PD et al (2011) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab 96:394–402
McClung MR et al (2013) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 24:227–235
Bone HG, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwinski E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Vittinghoff E, Daizadeh NS, Wang A, Dakin P, Wagman RB, Papapoulos S (2015) Ten years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension Trial. J Bone Miner Res (ASBMR Annual Meeting Abstracts) 30(Suppl 1):S471
Austin M et al (2012) Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 27:687–693
Kendler DL et al (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Recknor C et al (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121:1291–1299
Roux C et al (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54
Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A, Wang C, Wagman RB, Cummings SR (2016) Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 101(8):3163–3170
Roux C et al (2005) Recommendations for monitoring antiresorptive therapies in postmenopausal osteoporosis. Jt Bone Spine 72:26–31
Ravaud P et al (1999) Individual smallest detectable difference in bone mineral density measurements. J Bone Miner Res 14:1449–1456
Brown JP (2009) Comparison of the effect of Denosumab and Alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass. J Bone Miner Res 24:153–161
Ebina K et al (2016) The effects of switching daily teriparatide to oral bisphosphonates or denosumab in patients with primary osteoporosis. J Bone Miner Metab. https://doi.org/10.1007/s00774-015-0731-x
Sebba AI (2008) Significance of a decline in bone mineral density while receiving oral bisphosphonate treatment. Clin Therapeutics 30:443–451
Burnett-Bowie SAM (2009) Prediction of changes in bone mineral density in post menopausal women treated with one-weekly bisphosphonates. J Clin Endocrinol Metab 94:1097–1103
Eastell R (2015) Relationship between pretreatment rate of bone loss and bone density responce to once yearly Zoledronate.J Bone Miner. Res 30:570–574
Ominsky MS, Libanati C, Niu Q, Boyce RW, Kostenuik PJ, Wagman RB, Baron R, Dempster DW (2015) Sustained modeling-based bone formation during adulthood in Cynomolgus monkeys may contribute to continuous BMD gain with denosumab. J Bone Miner Res 7:1280–1289
Reid IR et al (2010) Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 25:2256–2265
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Laroche: punctual interventions in continuing medical education: Amgen, Lilly, MSD. C. Baradat: no conflict of interest. A. Ruyssen-Witrand: punctual interventions in continuing medical education: Pfeizer, MSD, Abbvie, UCB. Y. Degboe: no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards:CCP Toulouse II, 2014 – No. 393.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Laroche, M., Baradat, C., Ruyssen-Witrand, A. et al. Variability of Denosumab densitometric response in postmenopausal osteoporosis. Rheumatol Int 38, 461–466 (2018). https://doi.org/10.1007/s00296-018-3929-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-3929-0