Abstract
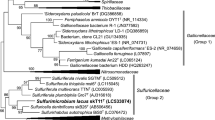
A bacterial isolate S23 capable of oxidizing thiosulfate was isolated from a sulfur spring. Strain S23 is gram-negative, aerobic, and motile. The G + C content of DNA is 61.4 mol%. The fatty acid composition and phylogenetic analysis of the 16S rRNA gene sequence of strain S23 showed that it is related to the members of the genus Comamonas, and most closely related to Comamonas testosteroni (99.9% sequence similarity). The isolate S23 exhibited thiosulfate oxidation under a mixotrophic growth condition. Polymerase chain reaction (PCR) using soxB-specific primers and DNA sequencing showed the presence of the soxB gene. This is the first report in Comamonas sp. showing thiosulfate oxidation under a mixotrophic growth condition.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA et al (1997) Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Appia-Ayme C, Little PJ, Matsumoto Y et al (2001) Cytochrome complex essential for photosynthetic oxidation of both thiosulfate and sulfide in Rhodovulum sulfidophilum. J Bacteriol 183:6107–6118
Brinkhoff T, Kuever J, Muyzer G, Jannasch HW (2005) Genus Thiomicrospira Kuenen and Veldkamp 1972, 253AL. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s manual of systematic bacteriology. Part B. The gammaproteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 193–199
Berglund F, Sorbo BH (1960) Turbidimetric analysis of inorganic sulfate in serum, plasma and urine. J Clin Lab Invest 12:147–150
Chou JH, Sheu SY, Lin KY et al (2007) Comamonas odontotermitis sp. nov., isolated from the gut of the termite Odontotermes formosanus. Int J Syst Evol Microbiol 57:887–891
Das SK, Mishra AK, Tindall BJ et al (1996) Oxidation of thiosulfate by a new bacterium, Bosea thiooxidans (strain BI-42) gen. nov., sp. nov.: Analysis of phylogeny based on chemotaxonomy and 16S ribosomal DNA sequencing. Int J Syst Bacteriol 46:981–987
Dayhoff MO, Schwartz RM, Orcutt BC (1978) A model of evolutionary change in proteins. In: Dayhoff MO (ed) Atlas of protein sequence structure, vol 5, suppl 3. National Biomedical Research Foundation, Washington, DC, pp 345–352
Elshahed MS, Savage KN, Oren A et al (2004) Haloferax sulfurifontis sp. nov., a halophilic archaeon isolated from a sulfide- and sulfur-rich spring. Int J Syst Evol Microbiol 54:2275–2279
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Felsenstein J (1989) Phylip-phylogenetic interference package (version 3.2). Cladistics 5:164–166
Ferrera I, Massana R, Casamayor EO et al (2004) High-diversity biofilm for the oxidation of sulfide-containing effluents. Appl Microbiol Biotechnol 64:726–734
Friedrich CG (1998) Physiology and genetics of sulfur oxidizing bacteria. Adv Microb Physiol 39:235–289
Friedrich CG, Rother D, Bardischewsky F et al (2001) Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Appl Environ Microbiol 67:2873–2882
Friedrich CG, Bardischewsky F, Rother D et al (2005) Prokaryotic sulfur oxidation. Curr Opin Microbiol 8:253–259
Gleen H, Quastel JH (1952) Sulfur metabolism in soil. Appl Microbiol 1:70–77
González JM, Covert JS, Whitman WB et al (2003) Silicibacter pomeroyi sp. nov. and Roseovarius nubinhibens sp. nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. Int J Syst Evol Microbiol 53:1261–1269
Hensen D, Sperling D, Truper HG et al (2006) Thiosulphate oxidation in the phototrophic sulphur bacterium Allochromatium vinosum. Mol Microbiol 62:794–810
Jorgensen BB (1990) The sulfur cycle of freshwater sediments: role of thiosulfate. Limnol Oceanogr 35:1329–1342
Kappler U, Friedrich CG, Trüper HG, Dahl C (2001) Evidence for two pathways of thiosulfate oxidation in Starkeya novella (formerly Thiobacillus novellus). Arch Microbiol 175:102–111
Kelly DP, Wood AP (2005) Genus Thiomonas Moreira and Amils 1997, 527VP. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s mannual of systematic bacteriology. Part C. The alpha, beta, delta and epsilon proteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 757–759
Kelly DP, Chambers LA, Trudinger PA (1969) Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem 41:898–901
Kelly DP, Wood AP, Stackebrandt E (2005) Genus Thiobacillus Beijerinck 1904b, 597AL. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s mannual of systematic bacteriology. Part C. The alpha, beta, delta, and epsilon proteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 764–771
Kletzin A, Urich T, Muller F et al (2004) Dissimilatory oxidation and reduction of elemental sulfur in thermophilic archaea. J Bioenerg Biomembr 36:77–91
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kuever J, Rainey FA, Widdel F (2005) Genus Desulfovibrio Kluver and van Niel, 397AL. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s mannual of systematic bacteriology. Part C. The alpha, beta, delta and epsilon proteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 926–938
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kuykendall LD, Roy MD, O’Neill JJ, Devine TE (1988) Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum. Int J Syst Bacteriol 38:358–361
Meade HM, Long SR, Ruvkun GB et al (1982) Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol 149:114–122
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G + C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Meyer B, Imhoff JF, Kuever J (2007) Molecular analysis of the distribution and phylogeny of the soxB gene among sulfur-oxidizing bacteria: Evolution of the Sox sulfur oxidation enzyme system. Environ Microbiol 9:2957–2977
Petri R, Podgorsek L, Imhoff JF (2001) Phylogeny and distribution of the soxB gene among thiosulfate-oxidizing bacteria. FEMS Microbiol Lett 197:171–178
Rainey FA, Kelly DP, Stackebrandt E et al (1999) A reevaluation of the taxonomy of Paracoccus denitrificans and a proposal for the combination Paracoccus pantotrophus comb. nov. Int J Syst Bacteriol 49:645–651
Schook LB, Berk RS (1978) Nutritional studies with Pseudomonas aeruginosa grown on inorganic sulfur sources. J Bacteriol 133:1377–1382
Sharma DP, Thomas C, Hall RH et al (1989) Significance of toxin coregulated pili as protective antigens of Vibrio cholerae in the infant mouse model. Vaccine 7:451–456
Sorokin DY, Teske A, Robertson LA, Kuenen JG (1999) Anaerobic oxidation of thiosulfate to tetrathionate by obligately heterotrophic bacteria belonging to the Pseudomonas stutzeri group. FEMS Microbiol Ecol 30:113–123
Sorokin DY, Tourova TP, Muyzer G (2005) Citreicella thiooxidans gen. nov., sp. nov., a novel lithoheterotrophic sulfur-oxidizing bacterium from the Black Sea. Syst Appl Microbiol 28:679–687
Strohl WR (2005) Genus Beggiatoa Trevisan 1842, 56AL. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s mannual of systematic bacteriology. Part B. The gammaproteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 148–161
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Trudinger PA (1967) Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol 93:550–559
Tuttle JH, Holmes PE, Jannasch HW (1974) Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol 99:1–14
Tuttle JH, Schwartz JH, Whited GM (1983) Some properties of thiosulfate-oxidizing enzyme from marine heterotroph 16B. Appl Environ Microbiol 46:438–445
Unz RF, Head IM (2005) Genus Thiothrix Winogradsky 1888, 39AL. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey’s mannual of systematic bacteriology. Part B. The gammaproteobacteria, vol 2, 2nd edn. Springer Science, New York, pp 131–142
Vitolins MI, Swaby RJ (1969) Activity of sulphur-oxidizing microorganisms in some Australian soils. Austr J Soil Res 7:171–183
Acknowledgments
We are grateful to Prof. R.M. Kroppenstedt and Dr. P. Schumann, DSMZ of Germany for their help in determining the G + C content and the fatty acid composition of the organism. This work was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, S.K., Narayan, K.D., Bandyopadhyay, S. et al. Thiosulfate Oxidation by Comamonas sp. S23 Isolated from a Sulfur Spring. Curr Microbiol 58, 516–521 (2009). https://doi.org/10.1007/s00284-009-9357-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9357-3