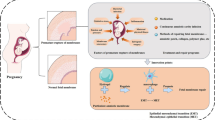
Abstract
Spontaneous preterm birth (PTB) and preterm pre-labor rupture of the membranes (pPROM) are major pregnancy complications. Although PTB and pPROM have common etiologies, they arise from distinct pathophysiologic pathways. Inflammation is a common underlying mechanism in both conditions. Balanced inflammation is required for fetoplacental growth; however, overwhelming inflammation (physiologic at term and pathologic at preterm) can lead to term and preterm parturition. A lack of effective strategies to control inflammation and reduce the risk of PTB and pPROM suggests that there are several modes of the generation of inflammation which may be dependent on the type of uterine tissue. The avascular fetal membrane (amniochorion), which provides structure, support, and protection to the intrauterine cavity, is one of the key contributors of inflammation. Localized membrane inflammation helps tissue remodeling during pregnancy. Two unique mechanisms that generate balanced inflammation are the progressive development of senescence (aging) and cyclic cellular transitions: epithelial to mesenchymal (EMT) and mesenchymal to epithelial (MET). The intrauterine build-up of oxidative stress at term or in response to risk factors (preterm) can accelerate senescence and promote a terminal state of EMT, resulting in the accumulation of inflammation. Inflammation degrades the matrix and destabilizes membrane function. Inflammatory mediators from damaged membranes are propagated via extracellular vesicles (EV) to maternal uterine tissues and transition quiescent maternal uterine tissues into an active state of labor. Membrane inflammation and its propagation are fetal signals that may promote parturition. This review summarizes the mechanisms of fetal membrane cellular senescence, transitions, and the generation of inflammation that contributes to term and preterm parturitions.




Similar content being viewed by others
References
Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R (2013) Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol 69(1):33–40. https://doi.org/10.1111/aji.12016
Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A et al (2015) Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am J Obstet Gynecol 213(6):830.e831-830.e819. https://doi.org/10.1016/j.ajog.2015.08.028
Agarwal A, Gupta S, Sharma RK (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28. https://doi.org/10.1186/1477-7827-3-28
Agrawal V, Hirsch E (2012) Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 17(1):12–19. https://doi.org/10.1016/j.siny.2011.09.001
Alijotas-Reig J, Llurba E, Gris JM (2014) Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta 35(4):241–248. https://doi.org/10.1016/j.placenta.2014.02.004
Ananth CV, Vintzileos AM (2006) Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 19(12):773–782. https://doi.org/10.1080/14767050600965882
Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC (2006) The Alabama preterm birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 195(3):803–808. https://doi.org/10.1016/j.ajog.2006.06.083
Antonakopoulos N, Iliodromiti Z, Mastorakos G, Iavazzo C, Valsamakis G, Salakos N et al (2018) Association between brain-derived neurotrophic factor (bdnf) levels in 2(nd) trimester amniotic fluid and fetal development. Mediat Inflamm 2018:8476217. https://doi.org/10.1155/2018/8476217
Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F (2002) Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol Reprod 67(6):1952–1958
Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N (2016) An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol 13(4):462–473. https://doi.org/10.1038/cmi.2015.22
Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG (2010a) The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail 12(8):819–825. https://doi.org/10.1093/eurjhf/hfq069
Barker DJ, Osmond C, Forsen TJ, Thornburg KL, Kajantie E, Eriksson JG (2013) Foetal and childhood growth and asthma in adult life. Acta Paediatr 102(7):732–738. https://doi.org/10.1111/apa.12257
Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG (2010b) The prenatal origins of lung cancer. II The placenta. Am J Hum Biol 22(4):512–516. https://doi.org/10.1002/ajhb.21041
Bartmann C, Segerer SE, Rieger L, Kapp M, Sutterlin M, Kammerer U (2014) Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol 71(2):109–119. https://doi.org/10.1111/aji.12185
Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH et al (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88(1):31–38. https://doi.org/10.2471/BLT.08.062554
Behnia F, Peltier M, Getahun D, Watson C, Saade G, Menon R (2016) High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med 29(22):3583-9. 1-7. https://doi.org/10.3109/14767058.2016.1139570
Behnia F, Peltier MR, Saade GR, Menon R (2015) Environmental pollutant polybrominated diphenyl ether, a flame retardant, induces primary amnion cell senescence 1. Am J Reprod Immunol. https://doi.org/10.1111/aji.12414
Behnia F, Sheller S, Menon R (2016a) Mechanistic differences leading to infectious and sterile inflammation. Am J Reprod Immunol 75(5):505–518. https://doi.org/10.1111/aji.12496
Behnia F, Sheller S, Menon R (2016b) Mechanistic differences leading to infectious and sterile inflammation 3. Am J Reprod Immunol. https://doi.org/10.1111/aji.12496
Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ et al (2015a) Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2015.05.041
Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ et al (2015b) Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol 213(3):359 e351-316. https://doi.org/10.1016/j.ajog.2015.05.041
Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ et al (2015c) Chorioamniotic membrane senescence: a signal for parturition? 1. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2015.05.041
Berghella V, Saccone G (2019) Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev 9:CD007235. https://doi.org/10.1002/14651858.CD007235.pub4
Bonney EA, Krebs K, Saade G, Kechichian T, Trivedi J, Huaizhi Y, Menon R (2016) Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta 43:26–34. https://doi.org/10.1016/j.placenta.2016.04.018
Boonkasidecha S, Kannan PS, Kallapur SG, Jobe AH, Kemp MW (2017) Fetal skin as a pro-inflammatory organ: evidence from a primate model of chorioamnionitis. PLoS One 12(9):e0184938. https://doi.org/10.1371/journal.pone.0184938
Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R (2014) HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One 9(12):e113799. https://doi.org/10.1371/journal.pone.0113799
Brosens I, Pijnenborg R, Vercruysse L, Romero R (2011) The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 204(3):193–201. https://doi.org/10.1016/j.ajog.2010.08.009
Bryant-Greenwood GD (1998) The extracellular matrix of the human fetal membranes: structure and function. Placenta 19(1):1–11
Buerzle W, Haller CM, Jabareen M, Egger J, Mallik AS, Ochsenbein-Koelble N et al (2013) Multiaxial mechanical behavior of human fetal membranes and its relationship to microstructure. Biomech Model Mechanobiol 12(4):747–762. https://doi.org/10.1007/s10237-012-0438-z
Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G et al (2009a) Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol 175(3):958–975. https://doi.org/10.2353/ajpath.2009.090156
Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G et al (2009b) Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol 175(3):958–975. https://doi.org/10.2353/ajpath.2009.090156
Buhimschi CS, Turan OM, Funai EF, Azpurua H, Bahtiyar MO, Turan S et al (2008) Fetal adrenal gland volume and cortisol/dehydroepiandrosterone sulfate ratio in inflammation-associated preterm birth. Obstet Gynecol 111(3):715–722. https://doi.org/10.1097/AOG.0b013e3181610294
Bukowski R, Sadovsky Y, Goodarzi H, Zhang H, Biggio JR, Varner M et al (2017) Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ 5:e3685. https://doi.org/10.7717/peerj.3685
Burstein R, Frankel S, Soule SD, Blumenthal HT (1973) Aging of the placenta: autoimmune theory of senescence. Am J Obstet Gynecol 116(2):271–276
Burton GJ (2009a) Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215(1):27–35. https://doi.org/10.1111/j.1469-7580.2008.00978.x
Burton GJ (2009b) Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215(1):27–35. https://doi.org/10.1111/j.1469-7580.2008.00978.x
Burton GJ, Jaunaiux E (2001) Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynecol Obstet Fertil 29(7-8):503–508
Burzle W, Mazza E, Moore JJ (2014) About puncture testing applied for mechanical characterization of fetal membranes. J Biomech Eng 136(11). https://doi.org/10.1115/1.4028446
Cai YJ, Huang L, Leung TY, Burd A (2014) A study of the immune properties of human umbilical cord lining epithelial cells. Cytotherapy 16(5):631–639. https://doi.org/10.1016/j.jcyt.2013.10.008
Campisi J (1997a) Aging and cancer: the double-edged sword of replicative senescence. J Am Geriatr Soc 45(4):482–488
Campisi J (1997b) The biology of replicative senescence. Eur J Cancer 33(5):703–709. https://doi.org/10.1016/S0959-8049(96)00058-5
Canzoneri BJ, Feng L, Grotegut CA, Bentley RC, Heine RP, Murtha AP (2013) The chorion layer of fetal membranes is prematurely destroyed in women with preterm premature rupture of the membranes. Reprod Sci. https://doi.org/10.1177/1933719113483009
Cao B, Stout MJ, Lee I, Mysorekar IU (2014) Placental microbiome and its role in preterm birth. Neoreviews 15(12):e537–e545. https://doi.org/10.1542/neo.15-12-e537
Cappelletti M, Presicce P, Lawson MJ, Chaturvedi V, Stankiewicz TE, Vanoni S et al (2017) Type I interferons regulate susceptibility to inflammation-induced preterm birth. JCI Insight 2(5):e91288. https://doi.org/10.1172/jci.insight.91288
Cha J, Hirota Y, Dey SK (2012) Sensing senescence in preterm birth. Cell Cycle 11(2):205–206. https://doi.org/10.4161/cc.11.2.18781
Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, Gomez R (2002) Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 186(6):1178–1182
Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F (2009) Inflammation and pregnancy. Reprod Sci 16(2):206–215. https://doi.org/10.1177/1933719108329095
Chambers ES, Akbar AN (2020) Can blocking inflammation enhance immunity during aging? J Allergy Clin Immunol 145(5):1323–1331. https://doi.org/10.1016/j.jaci.2020.03.016
Chavan AR, Griffith OW, Wagner GP (2017) The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr Opin Genet Dev 47:24–32. https://doi.org/10.1016/j.gde.2017.08.004
Chen J, Chen ZJ (2013) Regulation of NF-kappaB by ubiquitination. Curr Opin Immunol 25(1):4–12. https://doi.org/10.1016/j.coi.2012.12.005
Choi TG, Lee J, Ha J, Kim SS (2011) Apoptosis signal-regulating kinase 1 is an intracellular inducer of p38 MAPK-mediated myogenic signalling in cardiac myoblasts. Biochim Biophys Acta 1813(8):1412–1421. https://doi.org/10.1016/j.bbamcr.2011.04.001
Conde-Agudelo A, Romero R, Nicolaides KH (2020) Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2019.12.266
Condon JC, Jeyasuria P, Faust JM, Mendelson CR (2004) Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 101(14):4978–4983. https://doi.org/10.1073/pnas.0401124101
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6(12):2853–2868. https://doi.org/10.1371/journal.pbio.0060301
Cox LS, Redman C (2017) The role of cellular senescence in ageing of the placenta. Placenta 52:139–145. https://doi.org/10.1016/j.placenta.2017.01.116
DaSilva-Arnold S, James JL, Al-Khan A, Zamudio S, Illsley NP (2015) Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition. Placenta 36(12):1412–1418. https://doi.org/10.1016/j.placenta.2015.10.013
Devaskar SU, Thamotharan M (2007) Metabolic programming in the pathogenesis of insulin resistance. Rev Endocr Metab Disord 8(2):105–113. https://doi.org/10.1007/s11154-007-9050-4
Di Renzo GC, Roura LC (2006) Guidelines for the management of spontaneous preterm labor. J Perinat Med 34(5):359–366. https://doi.org/10.1515/JPM.2006.073
Di Sarno R, Raffone A, Saccone G (2019) Effects of progestogens in women with preterm premature rupture of membranes. Minerva Ginecol 71(2):121–124. https://doi.org/10.23736/S0026-4784.18.04335-6
Dimri GP, Campisi J (1994) Molecular and cell biology of replicative senescence. Cold Spring Harb Symp Quant Biol 59:67–73
Dixon CL, Richardson L, Sheller-Miller S, Saade G, Menon R (2018) A distinct mechanism of senescence activation in amnion epithelial cells by infection, inflammation, and oxidative stress. Am J Reprod Immunol 79(3). https://doi.org/10.1111/aji.12790
Dodd JM, Flenady VJ, Cincotta R, Crowther CA (2008) Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol 112(1):127–134. https://doi.org/10.1097/AOG.0b013e31817d0262
Dudley DJ (1999) Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol 180(1 Pt 3):S251–S256
Dunand C, Hoffmann P, Sapin V, Blanchon L, Salomon A, Sergent F et al (2014) Endocrine gland-derived endothelial growth factor (EG-VEGF) is a potential novel regulator of human parturition. Biol Reprod 91(3):73. https://doi.org/10.1095/biolreprod.114.119990
Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovsky M, Menon R (2016) Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod 22(2):143–157. https://doi.org/10.1093/molehr/gav074
Edey LF, O'Dea KP, Herbert BR, Hua R, Waddington SN, MacIntyre DA et al (2016) The local and systemic immune response to intrauterine lps in the prepartum mouse. Biol Reprod 95(6):125. https://doi.org/10.1095/biolreprod.116.143289
El KM, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM et al (2006) Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig 13(3):191–195. https://doi.org/10.1016/j.jsgi.2005.12.010
Elci E, Gunes Elci G, Sayan S (2020) Comparison of the accuracy and reliability of the AmniSure, AMNIOQUICK, and AL-SENSE tests for early diagnosis of premature rupture of membranes. Int J Gynaecol Obstet 149(1):93–97. https://doi.org/10.1002/ijgo.13097
Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M (2003) A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 163(5):2103–2111. https://doi.org/10.1016/S0002-9440(10)63567-5
Erlebacher A (2013) Immunology of the maternal-fetal interface. Annu Rev Immunol 31:387–411. https://doi.org/10.1146/annurev-immunol-032712-100003
Faye-Petersen OM (2008) The placenta in preterm birth. J Clin Pathol 61(12):1261–1275. https://doi.org/10.1136/jcp.2008.055244
Feng L, Allen TK, Marinello WP, Murtha AP (2019) Roles of progesterone receptor membrane component 1 in oxidative stress-induced aging in chorion cells. Reprod Sci 26(3):394–403. https://doi.org/10.1177/1933719118776790
Fernandez-Macias R, Martinez-Portilla RJ, Cerrillos L, Figueras F, Palacio M (2019) A systematic review and meta-analysis of randomized controlled trials comparing 17-alpha-hydroxyprogesterone caproate versus placebo for the prevention of recurrent preterm birth. Int J Gynaecol Obstet 147(2):156–164. https://doi.org/10.1002/ijgo.12940
Ferrari F, Facchinetti F, Saade G, Menon R (2016) Placental telomere shortening in stillbirth: a sign of premature senescence? J Matern Fetal Neonatal Med 29(8):1283–1288. https://doi.org/10.3109/14767058.2015.1046045
Figueiredo AS, Schumacher A (2016) The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 148(1):13–21. https://doi.org/10.1111/imm.12595
Finch CE (1992) Mechanisms in senescence: some thoughts in April 1990 3. Exp Gerontol 27(1):7–16
Fortunato SJ, Menon R, Swan KF, Lyden TW (1994) Organ culture of amniochorionic membrane in vitro. Am J Reprod Immunol 32(3):184–187
Fox H (1981) Invited review. A contemporary approach to placental pathology. Pathology 13(2):207–223
Freund A, Laberge RM, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23(11):2066–2075. https://doi.org/10.1091/mbc.E11-10-0884
Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA et al (2015) Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest 125(7):2808–2824. https://doi.org/10.1172/JCI78544
Garg M, Thamotharan M, Dai Y, Thamotharan S, Shin BC, Stout D, Devaskar SU (2012) Early postnatal caloric restriction protects adult male intrauterine growth-restricted offspring from obesity. Diabetes 61(6):1391–1398. https://doi.org/10.2337/db11-1347
Gnecco JS, Anders AP, Cliffel D, Pensabene V, Rogers LM, Osteen K, Aronoff DM (2017) Instrumenting a fetal membrane on a chip as emerging technology for preterm birth research. Curr Pharm Des. https://doi.org/10.2174/1381612823666170825142649
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371(9606):75–84. https://doi.org/10.1016/S0140-6736(08)60074-4
Golightly E, Jabbour HN, Norman JE (2011) Endocrine immune interactions in human parturition. Mol Cell Endocrinol 335(1):52–59. https://doi.org/10.1016/j.mce.2010.08.005
Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM, Vadillo-Ortega F (2013) Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod Sci 20(3):276–284. https://doi.org/10.1177/1933719112452473
Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D et al (2016) Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 75(1):3–7. https://doi.org/10.1111/aji.12443
Gomez-Lopez N, Romero R, Plazyo O, Schwenkel G, Garcia-Flores V, Unkel R et al (2017a) Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2017.08.008
Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG et al (2017b) A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci 24(6):934–953. https://doi.org/10.1177/1933719116675058
Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M (2014) Immune cells in term and preterm labor. Cell Mol Immunol 11(6):571–581. https://doi.org/10.1038/cmi.2014.46
Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F (2011) Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 205(3):235–224. https://doi.org/10.1016/j.ajog.2011.04.019
Gonzalez JM, Dong Z, Romero R, Girardi G (2011) Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One 6(11):e26877. https://doi.org/10.1371/journal.pone.0026877
Gryglewski RJ, Chlopicki S, Uracz W, Marcinkiewicz E (2001) Significance of endothelial prostacyclin and nitric oxide in peripheral and pulmonary circulation. Med Sci Monit 7(1):1–16
Hadley EE, Sheller-Miller S, Saade G, Salomon C, Mesiano S, Taylor RN et al (2018) Amnion epithelial cell derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2018.08.021
Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A et al (2012) Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 86(2):39. https://doi.org/10.1095/biolreprod.111.095505
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 154(1):8–20. https://doi.org/10.1159/000147748
Hayflick L (1961) The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res 23:14–20
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hinz M, Scheidereit C (2014) The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep 15(1):46–61. https://doi.org/10.1002/embr.201337983
Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK (2010a) Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 120(3):803–815. https://doi.org/10.1172/JCI40051
Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK (2010b) Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 120(3):803–815. https://doi.org/10.1172/JCI40051
Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM (2014) Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod 90(2):39. https://doi.org/10.1095/biolreprod.113.115428
Hoffman DR, Truong CT, Johnston JM (1986) The role of platelet-activating factor in human fetal lung maturation. Am J Obstet Gynecol 155(1):70–75. https://doi.org/10.1016/0002-9378(86)90081-5
Huszar G, Naftolin F (1984) The myometrium and uterine cervix in normal and preterm labor. N Engl J Med 311(9):571–581. https://doi.org/10.1056/NEJM198408303110905
Ilievski V, Lu SJ, Hirsch E (2007) Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci 14(4):315–320. https://doi.org/10.1177/1933719107302959
Iliodromiti Z, Antonakopoulos N, Sifakis S, Tsikouras P, Daniilidis A, Dafopoulos K et al (2012) Endocrine, paracrine, and autocrine placental mediators in labor. Hormones (Athens) 11(4):397–409. https://doi.org/10.14310/horm.2002.1371
Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD (2013) Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 191(11):5702–5713. https://doi.org/10.4049/jimmunol.1301604
Janzen C, Sen S, Lei MY, Gagliardi de Assumpcao M, Challis J, Chaudhuri G (2017) The role of epithelial to mesenchymal transition in human amniotic membrane rupture. J Clin Endocrinol Metab 102(4):1261–1269. https://doi.org/10.1210/jc.2016-3150
Jauniaux E, Poston L, Burton GJ (2006) Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 12(6):747–755. https://doi.org/10.1093/humupd/dml016
Jin J, Menon R (2017) Placental exosomes: A proxy to understand pregnancy complications. Am J Reprod Immunol. https://doi.org/10.1111/aji.12788
Jin J, Richardson L, Sheller-Miller S, Zhong N, Menon R (2018) Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. Placenta 67:15–23. https://doi.org/10.1016/j.placenta.2018.05.008
Joyce EM, Diaz P, Tamarkin S, Moore R, Strohl A, Stetzer B et al (2016) In-vivo stretch of term human fetal membranes. Placenta 38:57–66. https://doi.org/10.1016/j.placenta.2015.12.011
Keelan JA (2018) Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol 125:89–99. https://doi.org/10.1016/j.jri.2017.12.004
Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD (2003) Cytokines, prostaglandins and parturition--a review. Placenta 24(Suppl A):S33–S46
Kendal-Wright CE (2007a) Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci 14(8 Suppl):35–41. https://doi.org/10.1177/1933719107310763
Kendal-Wright CE (2007b) Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci 14(8 Suppl):35–41. https://doi.org/10.1177/1933719107310763
Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD (2008) Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta 29(3):255–265. https://doi.org/10.1016/j.placenta.2007.12.008
Kendal-Wright CE, Hubbard D, Gowin-Brown J, Bryant-Greenwood GD (2010) Stretch and inflammation-induced Pre-B cell colony-enhancing factor (PBEF/Visfatin) and Interleukin-8 in amniotic epithelial cells. Placenta 31(8):665–674. https://doi.org/10.1016/j.placenta.2010.06.007
Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, Yoon BH (2007) A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 197(3):292–295. https://doi.org/10.1016/j.ajog.2007.06.040
King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR (2007) Innate immune defences in the human uterus during pregnancy 6. Placenta 28(11-12):1099–1106. https://doi.org/10.1016/j.placenta.2007.06.002
Kramer MS, Papageorghiou A, Culhane J, Bhutta Z, Goldenberg RL, Gravett M et al (2012) Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol 206(2):108–112. https://doi.org/10.1016/j.ajog.2011.10.864
Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M et al (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33(12):982–990. https://doi.org/10.1016/j.placenta.2012.10.005
Kumar D, Novince R, Strohl A, Mercer BM, Mansour JM, Moore RM, Moore JJ (2009) A new methodology to measure strength of adherence of the fetal membrane components, amnion and the choriodecidua. Placenta 30(6):560–563. https://doi.org/10.1016/j.placenta.2009.03.014
Kumar D, Schatz F, Moore RM, Mercer BM, Rangaswamy N, Mansour JM et al (2011) The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta 32(3):206–213
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113(Pt 20):3613–3622
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196. https://doi.org/10.1038/nrm3758
Land WG (2015a) The role of damage-associated molecular patterns (DAMPSs) in human diseases: part ii: damps as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J 15(2):e157–e170
Land WG (2015b) The role of damage-associated molecular patterns in human diseases: part i - promoting inflammation and immunity. Sultan Qaboos Univ Med J 15(1):e9–e21
Langen ES, Sit A, Sherwin K, Lyell DJ, Blumenfeld YJ, El-Sayed YY (2018) A double-blind, randomized, placebo-controlled trial of 17 alpha-hydroxyprogesterone caproate in the management of preterm premature rupture of membranes. Am J Perinatol 35(8):779–784. https://doi.org/10.1055/s-0037-1617428
Lappas M (2013) NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod 89(1):14. https://doi.org/10.1095/biolreprod.113.110056
Lash GE (2015) molecular cross-talk at the feto-maternal interface. Cold Spring Harb Perspect Med 5(12). https://doi.org/10.1101/cshperspect.a023010
Lavu N, Richardson L, Radnaa E, Kechichian T, Urrabaz-Garza R, Sheller-Miller S et al (2019) Oxidative stress-induced downregulation of glycogen synthase kinase 3 beta in fetal membranes promotes cellular senescencedagger. Biol Reprod 101(5):1018–1030. https://doi.org/10.1093/biolre/ioz119
Lavu N, Sheller-Miller S, Kechichian T, Cayenne S, Bonney EA, Menon R (2020) Changes in mediators of pro-cell growth, senescence, and inflammation during murine gestation. Am J Reprod Immunol 83(3):e13214. https://doi.org/10.1111/aji.13214
Lim R, Barker G, Lappas M (2014) The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-kappaB-dependent signalling. Am J Reprod Immunol 71(5):401–417. https://doi.org/10.1111/aji.12229
Liu J, Dong P, Wang S, Li J (2019) Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from recurrent spontaneous abortion with normal and abnormal chromosome karyotypes. Biochem Biophys Res Commun 508(2):354–360. https://doi.org/10.1016/j.bbrc.2018.11.156
Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH (2017) The role of decidual immune cells on human pregnancy. J Reprod Immunol 124:44–53. https://doi.org/10.1016/j.jri.2017.10.045
Lockwood CJ (1994) Recent advances in elucidating the pathogenesis of preterm delivery, the detection of patients at risk, and preventative therapies. Curr Opin Obstet Gynecol 6(1):7–18
Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G et al (2007) Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem 40(11):793–797. https://doi.org/10.1016/j.clinbiochem.2007.03.004
Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J (1993) Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta 14(4):385–406
Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H et al (2015) The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol 212(4):487 e481-487 e411. https://doi.org/10.1016/j.ajog.2015.02.010
Martin LF, Richardson LS, da Silva MG, Sheller-Miller S, Menon R (2019) Dexamethasone induces primary amnion epithelial cell senescence through telomere-P21 associated pathwaydagger. Biol Reprod 100(6):1605–1616. https://doi.org/10.1093/biolre/ioz048
Masoro EJ (1995) Glucocorticoids and aging 1. Aging (Milano) 7(6):407–413
Mauri A, Ehret AE, Perrini M, Maake C, Ochsenbein-Kolble N, Ehrbar M et al (2015a) Deformation mechanisms of human amnion: Quantitative studies based on second harmonic generation microscopy. J Biomech 48(9):1606–1613. https://doi.org/10.1016/j.jbiomech.2015.01.045
Mauri A, Perrini M, Ehret AE, De Focatiis DS, Mazza E (2015b) Time-dependent mechanical behavior of human amnion: macroscopic and microscopic characterization. Acta Biomater 11:314–323. https://doi.org/10.1016/j.actbio.2014.09.012
McCabe ER, Carrino GE, Russell RB, Howse JL (2014) Fighting for the next generation: US Prematurity in 2030. Pediatrics 134(6):1193–1199. https://doi.org/10.1542/peds.2014-2541
McIntire RH, Ganacias KG, Hunt JS (2008) Programming of human monocytes by the uteroplacental environment. Reprod Sci 15(5):437–447. https://doi.org/10.1177/1933719107314065
Mendelson CR (2009) Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23(7):947–954. https://doi.org/10.1210/me.2009-0016
Mendelson CR, Montalbano AP, Gao L (2017) Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol 170:19–27. https://doi.org/10.1016/j.jsbmb.2016.09.006
Menon R (2008) Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand 87(6):590–600. https://doi.org/10.1080/00016340802005126
Menon R (2014) Oxidative stress damage as a detrimental factor in preterm birth pathology 14. Front Immunol 5:567. https://doi.org/10.3389/fimmu.2014.00567
Menon R (2016) Human fetal membranes at term: dead tissue or signalers of parturition? Placenta 44:1–5. https://doi.org/10.1016/j.placenta.2016.05.013
Menon R (2019) Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci 62(4):199–211. https://doi.org/10.5468/ogs.2019.62.4.199
Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M (2016a) Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 8(2):216–230. https://doi.org/10.18632/aging.100891
Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA et al (2014a) Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 184(6):1740–1751. https://doi.org/10.1016/j.ajpath.2014.02.011
Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR et al (2013) Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 8(12):e83416. https://doi.org/10.1371/journal.pone.0083416
Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN (2016b) Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update 22(5):535–560. https://doi.org/10.1093/humupd/dmw022
Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal P et al (2020) Protein profile changes in circulating placental extracellular vesicles in term and preterm births: a longitudinal study. Endocrinology 161(4). https://doi.org/10.1210/endocr/bqaa009
Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal PK et al (2019) Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology 160(2):249–275. https://doi.org/10.1210/en.2018-00836
Menon R, Fortunato SJ (2004) Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med 32(5):391–399. https://doi.org/10.1515/JPM.2004.134
Menon R, Fortunato SJ, Milne GL, Brou L, Carnevale C, Sanchez SC et al (2011) Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol 118(1):121–134. https://doi.org/10.1097/AOG.0b013e3182204eaa
Menon R, McIntyre JO, Matrisian LM, Fortunato SJ (2006) Salivary proteinase activity: a potential biomarker for preterm premature rupture of the membranes. Am J Obstet Gynecol 194(6):1609–1615. https://doi.org/10.1016/j.ajog.2006.02.052
Menon R, Mesiano S, Taylor RN (2017) Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. Front Endocrinol (Lausanne) 8:196. https://doi.org/10.3389/fendo.2017.00196
Menon R, Moore JJ (2020) Fetal membranes, not a mere appendage of the placenta, but a critical part of the fetal-maternal interface controlling parturition. Obstet Gynecol Clin N Am 47(1):147–162. https://doi.org/10.1016/j.ogc.2019.10.004
Menon R, N. N, Bredeson S, Polettini J (2015) Fetal Membranes: Potential Source of Preterm Birth Biomarkers. In: P. V. Preedy VR (ed) General Methods in Biomarker Research and Their Applications. Springer Science Publisher. (Reprinted from: Not in File), pp 483–529
Menon R, Papaconstantinou J (2016) p38 Mitogen activated protein kinase (MAPK): a new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin Ther Targets:1–16. https://doi.org/10.1080/14728222.2016.1216980
Menon R, Polettini J, Syed TA, Saade GR, Boldogh I (2014b) Expression of 8-oxoguanine glycosylase in human fetal membranes. Am J Reprod Immunol. https://doi.org/10.1111/aji.12220
Menon R, Richardson LS, Lappas M (2018) Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. https://doi.org/10.1016/j.placenta.2018.11.003
Menon R, Taylor BD (2019) Exploring inflammatory mediators in fetal and maternal compartments during human parturition. Obstet Gynecol 134(4):765–773. https://doi.org/10.1097/AOG.0000000000003470
Menon R, Taylor RN, Fortunato SJ (2010) Chorioamnionitis--a complex pathophysiologic syndrome. Placenta 31(2):113–120. https://doi.org/10.1016/j.placenta.2009.11.012
Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN (2012a) Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One 7(2):e31136. https://doi.org/10.1371/journal.pone.0031136
Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN (2012b) Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One 7(2):e31136. https://doi.org/10.1371/journal.pone.0031136
Mercer BM, Goldenberg RL, Meis PJ, Moawad AH, Shellhaas C, Das A et al (2000) The preterm prediction study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. the national institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol 183(3):738–745
Mercer BM, Lewis R (1997) Preterm labor and preterm premature rupture of the membranes. Diagnosis and management. Infect Dis Clin N Am 11(1):177–201
Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R (2002) Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87(6):2924–2930. https://doi.org/10.1210/jcem.87.6.8609
Mesiano S, Wang Y, Norwitz ER (2011) Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 18(1):6–19. https://doi.org/10.1177/1933719110382922
Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood G (2000) Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol 182(1 Pt 1):128–134
Miyazaki C, Moreno Garcia R, Ota E, Swa T, Oladapo OT, Mori R (2016) Tocolysis for inhibiting preterm birth in extremely preterm birth, multiple gestations and in growth-restricted fetuses: a systematic review and meta-analysis. Reprod Health 13:4. https://doi.org/10.1186/s12978-015-0115-7
Moco NP, Martin LF, Pereira AC, Polettini J, Peracoli JC, Coelho KI, da Silva MG (2013) Gene expression and protein localization of TLR-1, -2, -4 and -6 in amniochorion membranes of pregnancies complicated by histologic chorioamnionitis. Eur J Obstet Gynecol Reprod Biol 171(1):12–17. https://doi.org/10.1016/j.ejogrb.2013.07.036
Mogami H, Hari Kishore A, Akgul Y, Word RA (2017) Healing of Preterm Ruptured Fetal Membranes. Sci Rep 7(1):13139. https://doi.org/10.1038/s41598-017-13296-1
Mohan AR, Sooranna SR, Lindstrom TM, Johnson MR, Bennett PR (2007) The effect of mechanical stretch on cyclooxygenase type 2 expression and activator protein-1 and nuclear factor-kappaB activity in human amnion cells. Endocrinology 148(4):1850–1857. https://doi.org/10.1210/en.2006-1289
Montalbano AP, Hawgood S, Mendelson CR (2013) Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology 154(1):483–498. https://doi.org/10.1210/en.2012-1797
Mor G (2008) Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci 1127:121–128. https://doi.org/10.1196/annals.1434.006
Mor G, Cardenas I, Abrahams V, Guller S (2011) Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221:80–87. https://doi.org/10.1111/j.1749-6632.2010.05938.x
Mor G, Kwon JY (2015) Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol 213(4 Suppl):S131–S137. https://doi.org/10.1016/j.ajog.2015.06.039
Muglia LJ, Katz M (2010) The enigma of spontaneous preterm birth. N Engl J Med 362(6):529–535. https://doi.org/10.1056/NEJMra0904308
Murtha AP, Menon R (2015) Regulation of fetal membrane inflammation: a critical step in reducing adverse pregnancy outcome. Am J Obstet Gynecol 213(4):447–448. https://doi.org/10.1016/j.ajog.2015.07.008
Myatt L (2010) Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31(Suppl):S66–S69. https://doi.org/10.1016/j.placenta.2009.12.021
Myatt L, Cui X (2004) Oxidative stress in the placenta. Histochem. Cell Biol 122(4):369–382. https://doi.org/10.1007/s00418-004-0677-x
Myatt L, Sun K (2010) Role of fetal membranes in signaling of fetal maturation and parturition. Int J Dev Biol 54(2-3):545–553. https://doi.org/10.1387/ijdb.082771lm
Nancy P, Erlebacher A (2012) Epigenetic repression of chemokine expression at the maternal-fetal interface as a mechanism of feto-maternal tolerance. Med Sci (Paris) 28(12):1037–1039. https://doi.org/10.1051/medsci/20122812005
Negishi Y, Takahashi H, Kuwabara Y, Takeshita T (2018a) Innate immune cells in reproduction. J Obstet Gynaecol Res. https://doi.org/10.1111/jog.13759
Negishi Y, Takahashi H, Kuwabara Y, Takeshita T (2018b) Innate immune cells in reproduction. J Obstet Gynaecol Res 44(11):2025–2036. https://doi.org/10.1111/jog.13759
Nogami M, Kimura T, Seki S, Matsui Y, Yoshida T, Koike-Soko C et al (2016) A human amnion-derived extracellular matrix-coated cell-free scaffold for cartilage repair: in vitro and in vivo studies. Tissue Eng Part A 22(7-8):680–688. https://doi.org/10.1089/ten.TEA.2015.0285
Norwitz ER, Bonney EA, Snegovskikh VV, Williams MA, Phillippe M, Park JS, Abrahams VM (2015) Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med 5(11). https://doi.org/10.1101/cshperspect.a023143
Olson DM, Zaragoza DB, Shallow MC, Cook JL, Mitchell BF, Grigsby P, Hirst J (2003) Myometrial activation and preterm labour: evidence supporting a role for the prostaglandin F receptor--a review. Placenta 24(Suppl A):S47–S54. https://doi.org/10.1053/plac.2002.0938
Osman I, Young A, Jordan F, Greer IA, Norman JE (2006) Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig 13(2):97–103. https://doi.org/10.1016/j.jsgi.2005.12.002
Padron JG, Saito Reis CA, Kendal-Wright CE (2020) The role of danger associated molecular patterns in human fetal membrane weakening. Front Physiol 11:602. https://doi.org/10.3389/fphys.2020.00602
Park JS, Park CW, Lockwood CJ, Norwitz ER (2005) Role of cytokines in preterm labor and birth. Minerva Ginecol 57(4):349–366
Parmley T (1984) Placental senescence. Adv Exp Med Biol 176:127–132
Pavlicev M, Norwitz ER (2018) Human parturition: nothing more than a delayed menstruation. Reprod Sci 25(2):166–173. https://doi.org/10.1177/1933719117725830
Pei D, Shu X, Gassama-Diagne A, Thiery JP (2019) Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol 21(1):44–53. https://doi.org/10.1038/s41556-018-0195-z
Peltier MR (2003) Immunology of term and preterm labor. Reprod Biol Endocrinol 1:122. https://doi.org/10.1186/1477-7827-1-122
Petraglia F, Arcuri F, de Ziegler D, Chapron C (2012) Inflammation: a link between endometriosis and preterm birth. Fertil Steril 98(1):36–40. https://doi.org/10.1016/j.fertnstert.2012.04.051
Phillippe M, Sawyer MR, Edelson PK (2019) The telomere gestational clock: increasing short telomeres at term in the mouse. Am J Obstet Gynecol 220(5):496 e491-496 e498. https://doi.org/10.1016/j.ajog.2019.01.218
Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R (2015a) Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One 10(9):e0137188. https://doi.org/10.1371/journal.pone.0137188
Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R (2015b) Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One 10(9):e0137188. https://doi.org/10.1371/journal.pone.0137188
Polettini J, Richardson LS, Menon R (2018) Oxidative stress induces senescence and sterile inflammation in murine amniotic cavity. Placenta 63:26–31. https://doi.org/10.1016/j.placenta.2018.01.009
Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade GR, Menon R (2016) Screening of lysyl oxidase (LOX) and lysyl oxidase like (LOXL) enzyme expression and activity in preterm prelabor rupture of fetal membranes. J Perinat Med 44(1):99–109. https://doi.org/10.1515/jpm-2014-0337
Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA et al (2015) Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod 92(2):56. https://doi.org/10.1095/biolreprod.114.124420
Reinl EL, England SK (2015) Fetal-to-maternal signaling to initiate parturition 1. J Clin Invest 125(7):2569–2571. https://doi.org/10.1172/JCI82576
Richardson L, Dixon CL, Aguilera-Aguirre L, Menon R (2018) Oxidative stress-induced tgf-beta/tab1-mediated p38mapk activation in human amnion epithelial cells. Biol Reprod. https://doi.org/10.1093/biolre/ioy135
Richardson L, Gnecco J, Ding T, Osteen K, Rogers LM, Aronoff DM, Menon R (2019) Fetal membrane organ-on-chip: an innovative approach to study cellular interactions. Reprod Sci 1933719119828084. https://doi.org/10.1177/1933719119828084
Richardson L, Menon R (2018) Proliferative, migratory, and transition properties reveal metastate of human amnion cells. Am J Pathol 188(9):2004–2015. https://doi.org/10.1016/j.ajpath.2018.05.019
Richardson LS, Taylor RN, Menon R (2020) Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci Signal 13(618). https://doi.org/10.1126/scisignal.aay1486
Richardson LS, Vargas G, Brown T, Ochoa L, Sheller-Miller S, Saade GR et al (2017) Discovery and characterization of human amniochorionic membrane microfractures. Am J Pathol. https://doi.org/10.1016/j.ajpath.2017.08.019
Rinaldi SF, Makieva S, Saunders PT, Rossi AG, Norman JE (2017) Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol Hum Reprod 23(10):708–724. https://doi.org/10.1093/molehr/gax038
Robins JC, Marsit CJ, Padbury JF, Sharma SS (2011) Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front Biosci (Elite Ed) 3:690–700
Rodier F, Kim SH, Nijjar T, Yaswen P, Campisi J (2005) Cancer and aging: the importance of telomeres in genome maintenance. Int J Biochem Cell Biol 37(5):977–990. https://doi.org/10.1016/j.biocel.2004.10.012
Roh JS, Sohn DH (2018) Damage-associated molecular patterns in inflammatory diseases. Immune Netw 18(4):e27. https://doi.org/10.4110/in.2018.18.e27
Romero R, Dey SK, Fisher SJ (2014a) Preterm labor: one syndrome, many causes. Science 345(6198):760–765. https://doi.org/10.1126/science.1251816
Romero R, Dey SK, Fisher SJ (2014b) Preterm labor: one syndrome, many causes. Science 345(6198):760–765. https://doi.org/10.1126/science.1251816
Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O et al (2006) The preterm parturition syndrome. BJOG 113(Suppl 3):17–42. https://doi.org/10.1111/j.1471-0528.2006.01120.x
Romero R, Gotsch F, Pineles B, Kusanovic JP (2007) Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 65(12 Pt 2):S194–S202
Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ (2001) Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 10(5):297–300
Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C (2014) Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med 6(262):262–265. https://doi.org/10.1126/scitranslmed.3009871
Ruiz-Bonilla V, Perdiguero E, Gresh L, Serrano AL, Zamora M, Sousa-Victor P et al (2008) Efficient adult skeletal muscle regeneration in mice deficient in p38beta, p38gamma and p38delta MAP kinases. Cell Cycle 7(14):2208–2214. https://doi.org/10.4161/cc.7.14.6273
Saito S, Lin YC, Murayama Y, Hashimoto K, Yokoyama KK (2012) Human amnion-derived cells as a reliable source of stem cells 19. Curr Mol Med 12(10):1340–1349 CMM-EPUB-20120924-2 [pii]
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. https://doi.org/10.1093/epirev/mxs008
Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C (2015) Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation 12:21. https://doi.org/10.1186/s12974-015-0239-2
Sato BL, Collier ES, Vermudez SA, Junker AD, Kendal-Wright CE (2016) Human amnion mesenchymal cells are pro-inflammatory when activated by the Toll-like receptor 2/6 ligand, macrophage-activating lipoprotein-2. Placenta 44:69–79. https://doi.org/10.1016/j.placenta.2016.06.005
Schliefsteiner C, Peinhaupt M, Kopp S, Logl J, Lang-Olip I, Hiden U et al (2017) Human placental hofbauer cells maintain an anti-inflammatory m2 phenotype despite the presence of gestational diabetes mellitus. Front Immunol 8:888. https://doi.org/10.3389/fimmu.2017.00888
Schmitz T, Sentilhes L, Lorthe E, Gallot D, Madar H, Doret-Dion M et al (2019) Preterm premature rupture of the membranes: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol 236:1–6. https://doi.org/10.1016/j.ejogrb.2019.02.021
Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC (2018) Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J Immunol 201(2):325–334. https://doi.org/10.4049/jimmunol.1800058
Seferovic MD, Pace RM, Carroll M, Belfort B, Major AM, Chu DM et al (2019) Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. Am J Obstet Gynecol 221(2):146 e141-146 e123. https://doi.org/10.1016/j.ajog.2019.04.036
Sharp AN, Heazell AE, Crocker IP, Mor G (2010) Placental apoptosis in health and disease. Am J Reprod Immunol 64(3):159–169. https://doi.org/10.1111/j.1600-0897.2010.00837.x
Sheller-Miller S, Trivedi J, Yellon SM, Menon R (2019) Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci Rep 9(1):608. https://doi.org/10.1038/s41598-018-37002-x
Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R (2017) Damage-associated molecular pattern markers hmgb1 and cell-free fetal telomere fragments in oxidative-stressed amnion epithelial cell-derived exosomes. J Reprod Immunol 123:3–11. https://doi.org/10.1016/j.jri.2017.08.003
Shynlova O, Tsui P, Jaffer S, Lye SJ (2009) Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1):S2–S10. https://doi.org/10.1016/j.ejogrb.2009.02.044
Simhan HN, Canavan TP (2005) Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG 112(Suppl 1):32–37. https://doi.org/10.1111/j.1471-0528.2005.00582.x
Sinkey RG, Guzeloglu-Kayisli O, Arlier S, Guo X, Semerci N, Moore R et al (2020) Thrombin-induced decidual colony-stimulating factor-2 promotes abruption-related preterm birth by weakening fetal membranes. Am J Pathol 190(2):388–399. https://doi.org/10.1016/j.ajpath.2019.10.020
Smith R (1998) Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol 39(1-2):215–220
Smith R, Mesiano S, McGrath S (2002) Hormone trajectories leading to human birth. Regul Pept 108(2-3):159–164
Smith R, Nicholson RC (2007) Corticotrophin releasing hormone and the timing of birth. Front Biosci 12:912–918
Snegovskikh VV, Schatz F, Arcuri F, Toti P, Kayisli UA, Murk W et al (2009) Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and -2 expression: implications for infection-related preterm birth. Reprod. Sci 16(8):767–780. https://doi.org/10.1177/1933719109336623
Song D, Shi Y (2014) Immune system modifications and feto-maternal immune tolerance. Chin Med J 127(17):3171–3180
Southcombe J, Tannetta D, Redman C, Sargent I (2011) The immunomodulatory role of syncytiotrophoblast microvesicles 40. PLoS. One 6(5):e20245. https://doi.org/10.1371/journal.pone.0020245
Strauss JF 3rd. (2013) Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci 20(2):140–153. https://doi.org/10.1177/1933719111424454
Sun K, He P, Yang K (2002) Intracrine induction of 11beta-hydroxysteroid dehydrogenase type 1 expression by glucocorticoid potentiates prostaglandin production in the human chorionic trophoblast. Biol Reprod 67(5):1450–1455. https://doi.org/10.1095/biolreprod.102.005892
Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G et al (2015) The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol 194(4):1534–1544. https://doi.org/10.4049/jimmunol.1401536
Szekeres-Bartho J (2002) Immunological relationship between the mother and the fetus. Int Rev Immunol 21(6):471–495
Tattersall M, Engineer N, Khanjani S, Sooranna SR, Roberts VH, Grigsby PL et al (2008) Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction 135(4):569–579. https://doi.org/10.1530/REP-07-0461
Thornburg KL (2015) The programming of cardiovascular disease. J Dev Orig Health Dis 6(5):366–376. https://doi.org/10.1017/S2040174415001300
Trivedi S, Joachim M, McElrath T, Kliman HJ, Allred EN, Fichorova RN et al (2012) Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstet Gynecol 206(3):236 e231-238. https://doi.org/10.1016/j.ajog.2011.12.004
Vicovac L, Aplin JD (1996) Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 156(3):202–216. https://doi.org/10.1159/000147847
Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA et al (2012) The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet. Gynecol 206(2):119–123. https://doi.org/10.1016/j.ajog.2011.10.866
Vink JY, Qin S, Brock CO, Zork NM, Feltovich HM, Chen X et al (2016) A new paradigm for the role of smooth muscle cells in the human cervix. Am J Obstet Gynecol 215(4):478 e471-478 e411. https://doi.org/10.1016/j.ajog.2016.04.053
Vogel JP, Nardin JM, Dowswell T, West HM, Oladapo OT (2014) Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database Syst Rev (7):CD006169. https://doi.org/10.1002/14651858.CD006169.pub2
von Zglinicki T (2003) Replicative senescence and the art of counting 20. Exp Gerontol 38(11-12):1259–1264
von Zglinicki T, Martin-Ruiz CM (2005) Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5(2):197–203
Wadhwa PD, Culhane JF, Rauh V, Barve SS (2001) Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J 5(2):119–125
Wallack L, Thornburg K (2016) Developmental Origins, Epigenetics, and Equity: Moving Upstream. Matern Child Health J 20(5):935–940. https://doi.org/10.1007/s10995-016-1970-8
Whittle WL, Gibb W, Challis JR (2000) The characterization of human amnion epithelial and mesenchymal cells: the cellular expression, activity and glucocorticoid regulation of prostaglandin output. Placenta 21(4):394–401. https://doi.org/10.1053/plac.1999.0482
Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR et al (2018) Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol 79(6):e12820. https://doi.org/10.1111/aji.12820
Yellon SM (2017) Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod 96(1):13–23. https://doi.org/10.1095/biolreprod.116.142844
Zhang J, Shynlova O, Sabra S, Bang A, Briollais L, Lye SJ (2017) Immunophenotyping and activation status of maternal peripheral blood leukocytes during pregnancy and labour, both term and preterm. J Cell Mol Med 21(10):2386–2402. https://doi.org/10.1111/jcmm.13160
Funding
This study is funded by NIH/NIAD (1R21AI140249-01A1) and NIH/NICHD (5 R03 HD098469 02 and 1R01HD084532-01A1) to R Menon
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors report no conflict of interest
Additional information
This article is a contribution to the special issue on Preterm birth: Pathogenesis and clinical consequences revisited – Guest Editors: Anke Diemert and Petra Arck
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Menon, R., Behnia, F., Polettini, J. et al. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol 42, 431–450 (2020). https://doi.org/10.1007/s00281-020-00808-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-020-00808-x