Abstract
Purpose
Drug resistance is a serious problem in leukemia therapy. A novel purine nucleoside analogue, nelarabine, is available for the treatment of children with T cell acute lymphoblastic leukemia. We investigated the mechanisms of drug resistance to nelarabine.
Methods
Nelarabine-resistant cells were selected by stepwise and continuous exposure to nelarabine using the limiting dilution method in human B and T cell lymphoblastic leukemia cell lines. Expression analysis was performed using real-time polymerase chain reaction, and epigenetic analysis was performed using methylation-specific polymerase chain reaction and chromatin immunoprecipitation.
Results
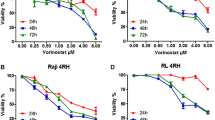
The RNA expression level for deoxycytidine kinase (dCK) was decreased in nelarabine-resistant leukemia cells. There were no differences between the parental and nelarabine-resistant leukemia cells in the methylation status of the promoter region of the dCK gene. In the chromatin immune precipitation assay, decreased acetylation of histones H3 and H4 bound to the dCK promoter was seen in the nelarabine-resistant cells when compared to the parental cells. Furthermore, treatment with a novel histone deacetylase inhibitor, vorinostat, promoted the cytotoxic effect of nelarabine along with increased expression of the dCK gene, and it increased acetylation of both histones H3 and H4 bound to the dCK promoter in nelarabine-resistant leukemia cells. The combination index showed that the effect of nelarabine and vorinostat was synergistic.
Conclusion
This study reports that nelarabine with vorinostat can promote cytotoxicity in nelarabine-resistant leukemia cells through epigenetic mechanisms.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, Janka-Schaub GE, Pieters R, Evans WE (2004) Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med 351:533–542. https://doi.org/10.1056/NEJMoa033513
Asano T (2020) Drug resistance in cancer therapy and the possible role of epigenetics. J Nippon Med Sch 87:244–251. https://doi.org/10.1272/jnms.JNMS.2020_87-508
Yamanishi M, Narazaki H, Asano T (2015) Melatonin overcomes resistance to clofarabine in two leukemic cell lines by increased expression of deoxycytidine kinase. Exp Hematol 43:207–214. https://doi.org/10.1016/j.exphem.2014.11.001
Homminga I, Zwaan CM, Manz CY, Parker C, Bantia S, Smits WK, Higginbotham F, Pieters R, Meijerink JP (2011) In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood 118:2184–2190. https://doi.org/10.1182/blood-2011-02-337840
Eden A, Gaudet F, Waghmare A, Jaenisch R (2003) Chromosomal instability and tumors promoted by DNA hypomethylation. Science 300:455. https://doi.org/10.1126/science.1083557
Dunsmore KP, Winter SS, Devidas M, Wood BL, Esiashvili N, Chen Z, Eisenberg N, Briegel N, Hayashi RJ, Gastier-Foster JM, Carroll AJ, Heerema NA, Asselin BL, Rabin KR, Zweidler-Mckay PA, Raetz EA, Loh ML, Schultz KR, Winick NJ, Carroll WL, Hunger SP (2020) Children’s Oncology Group AALL0434: a Phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol 38:3282–3293. https://doi.org/10.1200/JCO.20.00256
Asano T, Nakamura K, Fujii H, Horichi N, Ohmori T, Hasegawa K, Isoe T, Adachi M, Otake N, Fukunaga Y (2005) Altered expression of topoisomerase II α contributes to etoposide cross-resistant K562/MX2 cell line by aberrant methylation. Br J Cancer 92:1486–1492. https://doi.org/10.1038/sj.bjc.6602498
Asano T, Narazaki H, Fujita A (2017) Genome-wide DNA methylation profiling of CpG islands in a morpholino anthracycline derivative-resistant leukemia cell line: p38α as a novel candidate for resistance. Pharmacol Res Perspect 5:ePW00285. https://doi.org/10.1002/prp2.285
Nowak SJ, Corces VG (2004) Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet 20:214–220. https://doi.org/10.1016/j.tig.2004.02.007
Berger SL (2002) Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12:142–148. https://doi.org/10.1016/s0959-437x(02)00279-4
Beesley AH, Palmer ML, Ford J, Weller RE, Cummings AJ, Freitas JR, Firth MJ, Perera KU, de Klerk NH, Kees UR (2007) In vitro cytotoxicity of nelarabine, clofarabine and flavopiridol in paediatric acute lymphoblastic leukaemia. Br J Haematol 138:740–748. https://doi.org/10.1111/j.1365-2141.2007.06739.x
Zhao L, Wientjes MG, Au JL (2004) Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res 10:7994–8004. https://doi.org/10.1158/1078-0432.CCR-04-1087
Hubeek I, Stam RW, Peters GJ, Broekhuizen R, Meijerink JPP, van Wering ER, Gibson BES, Creutzig U, Zwaan CM, Cloos J, Kuik DJ, Pieters R, Kaspers GJL (2005) The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br J Cancer 93:1388–1394. https://doi.org/10.1038/sj.bjc.6602881
Stam RW, den Boer ML, Meijerink JP, Ebus ME, Peters GJ, Noordhuis P, Janka-Schaub GE, Armstrong SA, Korsmeyer SJ, Pieters R (2003) Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood 101:1270–1276. https://doi.org/10.1182/blood-2002-05-1600
Asano T, Tsutsuda-Asano A, Fukunaga Y (2009) Indomethacin overcomes doxorubicin resistance by decreasing intracellular content of glutathione and its conjugates with decreasing expression of gamma-glutamylcysteine synthetase via promoter activity in doxorubicin-resistant leukemia cells. Cancer Chemother Pharmacol 64:715–721. https://doi.org/10.1007/s00280-008-0920-6
Peters GJ, Hodzic J, Ortega B, Giovannetti E, Adema AD, Broekhuizen R, Kaspers GJ, Hubeek I (2010) Methylation specific PCR to characterize methylation of the promoter of deoxycytidine kinase. Nucleosides Nucleotides Nucleic Acids 29:408–413. https://doi.org/10.1080/15257771003730078
Candelaria M, de la Cruz-Hernandez E, Taja-Chayeb L, Perez-Cardenas E, Trejo-Becerril C, Gonzalez-Fierro A, Chavez-Blanco A, Soto-Reyes E, Dominguez G, Trujillo JE, Diaz-Chavez J, Duenas-Gonzalez A (2012) DNA methylation-independent reversion of gemcitabine resistance by hydralazine in cervical cancer cells. PLoS One 7:e29181. https://doi.org/10.1371/journal.pone.0029181
Yu CC, Pan SL, Chao SW, Liu SP, Hsu JL, Yang YC, Li TK, Huang WJ, Guh JH (2014) A novel small molecule hybrid of vorinostat and DACA displays anticancer activity against human hormone-refractory metastatic prostate cancer through dual inhibition of histone deacetylase and topoisomerase I. Biochem Pharmacol 90:320–330. https://doi.org/10.1016/j.bcp.2014.06.001
Funding
This study was supported in part by grants from JSPS KAKENHI (Grant-in-Aid for Scientific Research C: 16K10044), the Promotion and Mutual Aid Corporation for Private Schools of Japan and the Science Research Promotion Fund.
Author information
Authors and Affiliations
Contributions
TA and YI designed and conceived the in vitro experiments. HN, AF and KY performed the in vitro experiments, and KY wrote the first draft of the manuscript. TA and KY wrote the paper. The authors analyzed the data, and reviewed, edited and approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board of the Nippon Medical School Chiba Hokusoh Hospital approved this study.
Consent to participate
Not applicable.
Consent for publication
The authors approved the final version of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
280_2021_4373_MOESM3_ESM.tif
Supplementary file3. Supplemental Fig. 1. The methylation status of the dCK promoter in BALL, Molt4, NALM6 and SKW3 parental and nelarabine-resistant cells. Methylated and unmethylated dCK promoters were evaluated by methylation-specific PCR using qPCR. Relative values (ratio) were calculated based on the expression level of dCK in BALL parental cells (set as 1). Data are shown as the average from two independent experiments. Identical methylation statuses were observed in the CpG islands of all examined dCK promoters between the parental and nelarabine-resistant cells. (TIF 8270 kb)
280_2021_4373_MOESM4_ESM.tif
Supplementary file4. Supplemental Fig. 2. Combination index (CI)-fraction affected (Fa) curves for BALL/P, BALL/NEL, Molt4/P, Molt4/NEL, NALM6/P, NALM6/NEL, SKW3/P, and SLW3/NEL cells in combination therapy with nelarabine and vorinostat. The drug concentration ratios were as follows: nelarabine+vorinostat (1000:1). CI values less than 1 indicate a synergistic effect, CI values greater than 1 indicate an antagonistic effect and CI values equal to 1 indicate an additive effect. (TIF 8270 kb)
280_2021_4373_MOESM5_ESM.tif
Supplementary file5. Supplemental Fig. 3. Cytotoxicity in BALL, Molt4, NALM6 and SKEW3 parental and nelarabine-resistant cells with or without 5-aza-2′-deoxycytidine treatment. Leukemia cells were incubated with various concentrations of nelarabine for 72 h with or without 0.2 μmol/L of 5-aza-2′-deoxycytidine, and cytotoxicity was evaluated by trypan blue dye exclusion assay. Data are shown as the mean ± standard deviation from three independent experiments. (TIF 33981 kb)
Rights and permissions
About this article
Cite this article
Yoshida, K., Fujita, A., Narazaki, H. et al. Drug resistance to nelarabine in leukemia cell lines might be caused by reduced expression of deoxycytidine kinase through epigenetic mechanisms. Cancer Chemother Pharmacol 89, 83–91 (2022). https://doi.org/10.1007/s00280-021-04373-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04373-4