Abstract
Purpose
The repeated use of doxorubicin is limited due to dose-limiting cardiac toxicity. Pegylated liposomal doxorubicin (PEG-LD, Duomeisu) has a reduced cardiac toxicity. This phase I study aimed to investigate the maximum tolerated doses (MTDs) and dose-limiting toxicities (DLTs) of the PEG-LD and cisplatin combination in patients with metastatic and recurrent osteosarcoma.
Methods
Patients were given PEG-LD at a dose of 40, 50, or 60 mg/m2 on day 1 of each 21-day cycle, according to a 3 + 3 approach for dose escalation. Cisplatin was administered as a fixed dose of 100 mg/m2 for every cycle. Toxicities and tumor response were observed.
Results
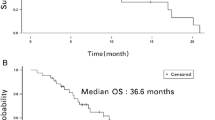
A total of 15 patients were enrolled in this trial, and nine of the patients had received prior doxorubicin. The MTD of PEG-LD was reached at 50 mg/m2 in this regimen, with neutropenic fever and stomatitis as DTLs. The main adverse event (AE) was myelosuppression. The most common non-hematological AEs were vomiting, hypoproteinemia, stomatitis and transient sinus arrhythmia. Grade 3–4 toxicity was neutropenia, leukopenia, thrombocytopenia, anemia and stomatitis in the whole cohort. All the AEs were relieved after symptomatic and supportive treatment. Totally, the overall response rate was 13.3% and disease control rate was 66.7%. For the six patients who have not received prior doxorubicin, one partial response and five stable diseases were observed.
Conclusion
We provide the data showing that PEG-LD 50 mg/m2 combined with cisplatin 100 mg/m2 demonstrated an acceptable safety profile and promising clinical activity in advanced osteosarcoma, which merits further evaluation in phase II studies.
Trial registration
ChiCTR1900021550.

Similar content being viewed by others
Availability of data and material
Data supporting the results reported in this study are not publicly available but are accessible from the corresponding author on reasonable request.
References
Mirabello L, Troisi RJ, Savage SA (2009) International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 125:229–234. https://doi.org/10.1002/ijc.24320
Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S (2003) Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol 14:1126–1134. https://doi.org/10.1093/annonc/mdg286
Nagarajan R, Neglia JP, Clohisy DR, Robison LL (2002) Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol 20:4493–4501. https://doi.org/10.1200/JCO.2002.09.006
Meyers PA, Heller G, Healey JH, Huvos A, Applewhite A, Sun M, LaQuaglia M (1993) Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol 11:449–453. https://doi.org/10.1200/JCO.1993.11.3.449
Luetke A, Meyers PA, Lewis I, Juergens H (2014) Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev 40:523–532. https://doi.org/10.1016/j.ctrv.2013.11.006
Leary SE, Wozniak AW, Billups CA, Wu J, McPherson V, Neel MD, Rao BN, Daw NC (2013) Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children’s Research Hospital experience. Cancer 119:2645–2653. https://doi.org/10.1002/cncr.28111
Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O 3rd, Widemann BC, Zwerdling T, Bomgaars L, Langevin AM, Grier HE, Weigel B, Blaney SM, Gorlick R, Janeway KA (2016) Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: learning from the past to move forward. J Clin Oncol 34:3031–3038. https://doi.org/10.1200/JCO.2015.65.5381
Bramwell VH, Burgers M, Sneath R, Souhami R, van Oosterom AT, Voute PA, Rouesse J, Spooner D, Craft AW, Somers R et al (1992) A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol 10:1579–1591. https://doi.org/10.1200/JCO.1992.10.10.1579
Huang YJ, He AN, Sun YJ, Shen Z, Min DL, Yao Y (2015) Continuous-infusion ifosfamide and doxorubicin combination as second-line chemotherapy for recurrent or refractory osteosarcoma patients in China: a retrospective study. Asian Pac J Cancer Prev 16:2391–2395. https://doi.org/10.7314/apjcp.2015.16.6.2391
Skubitz KM (2003) Phase II trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Invest 21:167–176. https://doi.org/10.1081/cnv-120016412
Schmitt CJ, Dietrich S, Ho AD, Witzens-Harig M (2012) Replacement of conventional doxorubicin by pegylated liposomal doxorubicin is a safe and effective alternative in the treatment of non-Hodgkin’s lymphoma patients with cardiac risk factors. Ann Hematol 91:391–397. https://doi.org/10.1007/s00277-011-1308-y
Duggan ST, Keating GM (2011) Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs 71:2531–2558. https://doi.org/10.2165/11207510-000000000-00000
Ferrandina G, Corrado G, Licameli A, Lorusso D, Fuoco G, Pisconti S, Scambia G (2010) Pegylated liposomal doxorubicin in the management of ovarian cancer. Ther Clin Risk Manag 6:463–483. https://doi.org/10.2147/TCRM.S3348
Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, van Oosterom AT, Clemons MJ, Kamby C, Hermans C, Whittaker J, Donato di Paola E, Verweij J, Nielsen S (2001) Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 37:870–877. https://doi.org/10.1016/s0959-8049(01)00050-8
Li Y, Huang Z, Shan HC, Xu HR, Sun Y, Niu XH (2019) Single-center, non-randomized controlled study of doxorubicin and doxorubicin in neoadjuvant chemotherapy for osteosarcoma. Chin J Cancer Prev Treat 26:1124–1128. https://doi.org/10.16073/j.cnki.cjcpt.2019.15.013
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, Group CBCS (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15:440–449. https://doi.org/10.1093/annonc/mdh097
Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A (2000) Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 11:1029–1033. https://doi.org/10.1023/a:1008365716693
Al-Batran SE, Guntner M, Pauligk C, Scholz M, Chen R, Beiss B, Stopatschinskaja S, Lerbs W, Harbeck N, Jager E (2010) Anthracycline rechallenge using pegylated liposomal doxorubicin in patients with metastatic breast cancer: a pooled analysis using individual data from four prospective trials. Br J Cancer 103:1518–1523. https://doi.org/10.1038/sj.bjc.6605961
Sparano JA, Makhson AN, Semiglazov VF, Tjulandin SA, Balashova OI, Bondarenko IN, Bogdanova NV, Manikhas GM, Oliynychenko GP, Chatikhine VA, Zhuang SH, Xiu L, Yuan Z, Rackoff WR (2009) Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III study. J Clin Oncol 27:4522–4529. https://doi.org/10.1200/JCO.2008.20.5013
Ansari L, Shiehzadeh F, Taherzadeh Z, Nikoofal-Sahlabadi S, Momtazi-Borojeni AA, Sahebkar A, Eslami S (2017) The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther 24:189–193. https://doi.org/10.1038/cgt.2017.9
Lyass O, Hubert A, Gabizon AA (2001) Phase I study of doxil-cisplatin combination chemotherapy in patients with advanced malignancies. Clin Cancer Res 7:3040–3046
Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N, Bernstein M, Link MP (2002) Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res 8:413–418
Lyseng-Williamson KA, Duggan ST, Keating GM (2013) Pegylated liposomal doxorubicin: a guide to its use in various malignancies. BioDrugs 27:533–540. https://doi.org/10.1007/s40259-013-0070-1
Acknowledgements
We are grateful to our patients and their families for participating in this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
XZW, XHN and XZ designed the study. XZW, QZP, BSX, WX, DSW, JJZ, HRX, ZH and XZ conducted the study and collected the data. XZW, QZP, XHN and XZ analyzed the data and interpreted the results. XZW, QZP, XHN and XZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no completing interests.
Ethical approval
The study was approved by the Scientific and Ethics Committees of Sun Yat-sen University Cancer Center (Number B2018-102-05).
Consent to participate
Each patient signed a written informed consent before participating to this study (Trial Registration: ChiCTR, ChiCTR1900021550).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, Xz., Pan, Qz., Xu, Bs. et al. Phase I study of pegylated liposomal doxorubicin and cisplatin in patients with advanced osteosarcoma. Cancer Chemother Pharmacol 89, 209–215 (2022). https://doi.org/10.1007/s00280-021-04371-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04371-6