Abstract
Purpose
Metronomic chemotherapy (MC) is a promising approach where, in contrast to the conventional maximal tolerated dose (MTD) strategy, regular fractionated doses of the drug are used. This approach has proven its efficacy, although drug dosing and scheduling are often chosen empirically. Pharmacokinetic/pharmacodynamic (PK/PD) models provide a way to choose optimal protocols with computational methods. Existing models are usually too complicated and are valid for only a subset of drug schedules. To address this issue, we propose herein a simple model that can describe MC and MTD regimens simultaneously.
Methods
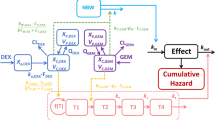
The minimal model comprises tumor suppression due to antiangiogenic drug effect together with a cell-kill term, responsible for its cytotoxicity. The model was tested on data obtained on tumor-bearing mice treated with gemcitabine in ether MTD, MC, or combined (MTD + MC) regimens.
Results
We conducted a number of tests in which data were divided in various ways into training and validation sets. The model successfully described different trends in the MTD and MC regimens. With parameters obtained by fitting the model to MTD data, the simulations correctly predicted trends in both the MC and combined therapy groups.
Conclusion
Our results demonstrate that the proposed model presents a minimal yet efficient tool for modeling outcomes in different treatment regimens in mice. We hope that this model has the potential for use in clinical practice in the development of patient-specific chemotherapy scheduling protocols based on observed treatment response.





Similar content being viewed by others
Data availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
André N, Carré M, Pasquier E (2014) Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol 11(7):413
André N, Tsai K, Carré M, Pasquier E (2017) Metronomic chemotherapy: direct targeting of cancer cells after all? Trends Cancer 3(5):319–325
Barbolosi D, Ciccolini J, Lacarelle B, Barlési F, André N (2016) Computational oncology-mathematical modelling of drug regimens for precision medicine. Nat Rev Clin Oncol 13(4):242
Barbolosi D, Ciccolini J, Meille C, Elharrar X, Faivre C, Lacarelle B, André N, Barlesi F (2014) Metronomics chemotherapy: time for computational decision support. Cancer Chemother Pharmacol 74(3):647–652
Benzekry S, Pasquier E, Barbolosi D, Lacarelle B, Barlési F, André N, Ciccolini J (2015) Metronomic reloaded: Theoretical models bringing chemotherapy into the era of precision medicine. Sem Cancer Biol 35(1):53–61
Briasoulis E, Aravantinos G, Kouvatseas G, Pappas P, Biziota E, Sainis I, Makatsoris T, Varthalitis I, Xanthakis I, Vassias A et al (2013) Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. Bmc Cancer 13(1):263
Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60(7):1878–1886
Cham K, Baker J, Takhar K, Flexman J, Wong M, Owen D, Yung A, Kozlowski P, Reinsberg S, Chu E et al (2010) Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer 103(1):52–60
Ciccolini J, Barbolosi D, Meille C, Lombard A, Serdjebi C, Giacometti S, Padovani L, Pasquier E, André N (2017) Pharmacokinetics and pharmacodynamics-based mathematical modeling identifies an optimal protocol for metronomic chemotherapy. Cancer Res 77(17):4723–4733
Ciccolini J, Serdjebi C, Peters GJ, Giovannetti E (2016) Pharmacokinetics and pharmacogenetics of gemcitabine as a mainstay in adult and pediatric oncology: an eortc-pamm perspective. Cancer Chemother Pharmacol 78(1):1–12
Faivre C, Barbolosi D, Pasquier E, André N (2013) A mathematical model for the administration of temozolomide: comparative analysis of conventional and metronomic chemotherapy regimens. Cancer Chemother Pharmacol 71(4):1013–1019
Fox J (2015) Applied regression analysis and generalized linear models. Sage Publications, New York
Francia G, Shaked Y, Hashimoto K, Sun J, Yin M, Cesta C, Xu P, Man S, Hackl C, Stewart J et al (2012) Low-dose metronomic oral dosing of a prodrug of gemcitabine (ly2334737) causes antitumor effects in the absence of inhibition of systemic vasculogenesis. Mol Cancer Ther 11(3):680–689
Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L (1999) Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res 59(19):4770–4775
Hao YB, Yi SY, Ruan J, Zhao L, Nan KJ (2014) New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett 354(2):220–226
Houy N, Le Grand F (2018) Optimal dynamic regimens with artificial intelligence: The case of temozolomide. PloS One 13(6):e0199076
Kodama M, Kodama T (1975) Enhancing effect of hydrocortisone on hematogenous metastasis of ehrlich ascites tumor in mice. Cancer Res 35(4):1015–1021
Ledzewicz U, Schättler H (2014) A review of optimal chemotherapy protocols: from MTD towards metronomic therapy. Math Model Nat Phenomena 9(4):131–152
Mager DE, Wyska E, Jusko WJ (2003) Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 31(5):510–518
Rocchetti M, Germani M, Del Bene F, Poggesi I, Magni P, Pesenti E, De Nicolao G (2013) Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth after administration of an anti-angiogenic agent, bevacizumab, as single-agent and combination therapy in tumor xenografts. Cancer Chemother Pharmacol 71(5):1147–1157
Romiti A, Falcone R, Roberto M, Marchetti P (2017) Current achievements and future perspectives of metronomic chemotherapy. Invest New Drugs 35(3):359–374
Schättler H, Ledzewicz U, Amini B (2016) Dynamical properties of a minimally parameterized mathematical model for metronomic chemotherapy. J Math Biol 72(5):1255–1280
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, Germani M, Poggesi I, Rocchetti M (2004) Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res 64(3):1094–1101
Veerman G, van Haperen VR, Vermorken JB, Noordhuis P, Braakhuis BJ, Pinedo HM, Peters GJ (1996) Antitumor activity of prolonged as compared with bolus administration of 2, 2-difluorodeoxycytidine in vivo against murine colon tumors. Cancer Chemother Pharmacol 38(4):335–342
Verschuur A, Heng-Maillard MA, Dory-Lautrec P, Truillet R, Jouve E, Chastagner P, Leblond P, Aerts I, Honoré S, Entz-Werle N et al (2018) Metronomic four-drug regimen has anti-tumor activity in pediatric low-grade glioma; the results of a phase ii clinical trial. Front Pharmacol 9:950
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J et al (2020) Scipy 1.0: fundamental algorithms for scientific computing in python. Nat Methods 17(3):261–272
Yamazaki S, Skaptason J, Romero D, Lee JH, Zou HY, Christensen JG, Koup JR, Smith BJ, Koudriakova T (2008) Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug Metab Dispos 36(7):1267–1274
Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12(1):1–10
Yapp DT, Wong MQ, Kyle AH, Valdez SM, Tso J, Yung A, Kozlowski P, Owen DA, Buczkowski AK, Chung SW et al (2016) The differential effects of metronomic gemcitabine and antiangiogenic treatment in patient-derived xenografts of pancreatic cancer: treatment effects on metabolism, vascular function, cell proliferation, and tumor growth. Angiogenesis 19(2):229–244
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Terterov, I.N., Chubenko, V.A., Knyazev, N.A. et al. Minimal PK/PD model for simultaneous description of the maximal tolerated dose and metronomic treatment outcomes in mouse tumor models. Cancer Chemother Pharmacol 88, 867–878 (2021). https://doi.org/10.1007/s00280-021-04326-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04326-x