Abstract
Purpose
The association between the pharmacokinetics and pharmacodynamics of regorafenib, a multiple tyrosine kinase inhibitor, remains unclear. This study assessed the trough plasma concentrations (Ctrough) of regorafenib and its N-oxide (M2) and N-oxide/desmethyl (M5) metabolites, and evaluated the associations among these levels, adverse events, and pharmacokinetic-related genetic polymorphisms in patients with metastatic colorectal cancer.
Methods
The Ctrough levels of regorafenib and its metabolites were assessed in a single-center, prospective, observational study, 7 days after the initial treatment. The correlation between those values and adverse events was then examined. In addition, the genetic polymorphisms of ABCG2, SLCO1B1, and UGT1A9 were determined and evaluated for associations with the levels of regorafenib, M2, and M5.
Results
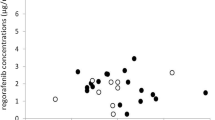
We analyzed 43 patients who received regorafenib 40–120 mg/day; among them, 35 patients started at 120 mg/day. With regard to bilirubin increase, the Ctrough values of regorafenib were significantly higher in the group with grade ≥ 2 than in groups with grades 0 and 1 (p = 0.010). The M5 Ctrough levels were significantly associated with the severity of hypertension or rash (p < 0.05). In a multivariate analysis, the M5 Ctrough values and age were significant predictors of severe rash. Lastly, significant differences were noted in the M5 concentration-to-dose ratio values between the patients with ABCG2 421A/A and ABCG2 421C/A or C/C polymorphisms (p = 0.035).
Conclusion
This study showed that the Ctrough of regorafenib was associated with bilirubin increase, and also clarified for the first time that the Ctrough of M5 was significantly correlated with hypertension and severe rash.


Similar content being viewed by others
References
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129(1):245–255. https://doi.org/10.1002/ijc.25864
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312. https://doi.org/10.1016/S0140-6736(12)61900-X
Yoshino T, Komatsu Y, Yamada Y, Yamazaki K, Tsuji A, Ura T, Grothey A, Van Cutsem E, Wagner A, Cihon F, Hamada Y, Ohtsu A (2015) Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Investig New Drugs 33(3):740–750. https://doi.org/10.1007/s10637-014-0154-x
Yamaguchi K, Komatsu Y, Satoh T, Uetake H, Yoshino T, Nishida T, Yamazaki N, Takikawa H, Morimoto T, Chosa M, Sunaya T, Hamada Y, Muro K, Sugihara K (2019) Large-scale, prospective observational study of regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist 24(7):e450–e457. https://doi.org/10.1634/theoncologist.2018-0377
Van Cutsem E, Martinelli E, Cascinu S, Sobrero A, Banzi M, Seitz JF, Barone C, Ychou M, Peeters M, Brenner B, Hofheinz RD, Maiello E, André T, Spallanzani A, Garcia-Carbonero R, Arriaga YE, Verma U, Grothey A, Kappeler C, Miriyala A, Kalmus J, Falcone A, Zaniboni A (2019) Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, Open-Label Phase IIIb CONSIGN Study. Oncologist 24(2):185–192. https://doi.org/10.1634/theoncologist.2018-0072
Maeda A, Irie K, Ando H, Hasegawa A, Taniguchi H, Kadowaki S, Muro K, Tajika M, Aoki M, Inaguma K, Kajita M, Fujimura A, Fukushima S (2019) Associations among regorafenib concentrations, severe adverse reactions, and ABCG2 and OATP1B1 polymorphisms. Cancer Chemother Pharmacol 83(1):107–113. https://doi.org/10.1007/s00280-018-3710-9
Taguchi D, Inoue M, Fukuda K, Yoshida T, Shimazu K, Fujita K, Okuyama H, Matsuhashi N, Tsuji A, Yoshida K, Miura M, Shibata H (2019) Therapeutic drug monitoring of regorafenib and its metabolite M5 can predict treatment efficacy and the occurrence of skin toxicities. Int J Clin Oncol. https://doi.org/10.1007/s10147-019-01593-w
Zopf D, Fichtner I, Bhargava A, Steinke W, Thierauch KH, Diefenbach K, Wilhelm S, Hafner FT, Gerisch M (2016) Pharmacologic activity and pharmacokinetics of metabolites of regorafenib in preclinical models. Cancer Med 5(11):3176–3185. https://doi.org/10.1002/cam4.883
Suzuki T, Sukawa Y, Imamura CK, Masuishi T, Satake H, Kumekawa Y, Funakoshi S, Kotaka M, Horie Y, Kawai S, Okuda H, Terazawa T, Kondoh C, Kato K, Yoshimura K, Ishikawa H, Hamamoto Y, Boku N, Takaishi H, Kanai T (2019) A Phase II Study of Regorafenib With a Lower Starting Dose in Patients With Metastatic Colorectal Cancer: Exposure-Toxicity Analysis of Unbound Regorafenib and Its Active Metabolites (RESET Trial). Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2019.10.004
Kubota Y, Fujita KI, Takahashi T, Sunakawa Y, Ishida H, Hamada K, Ichikawa W, Tsunoda T, Shimada K, Masuo Y, Kato Y, Sasaki Y (2020) Higher Systemic Exposure to Unbound Active Metabolites of Regorafenib Is Associated With Short Progression-Free Survival in Colorectal Cancer Patients. Clin Pharmacol Ther 108(3):586–595. https://doi.org/10.1002/cpt.1810
Miura K, Satoh M, Kinouchi M, Yamamoto K, Hasegawa Y, Philchenkov A, Kakugawa Y, Fujiya T (2014) The preclinical development of regorafenib for the treatment of colorectal cancer. Expert Opin Drug Discov 9(9):1087–1101. https://doi.org/10.1517/17460441.2014.924923
Li YH, Lin QM, Pang NH, Zhang XD, Huang HL, Cai JP, Hu GX (2019) Functional characterization of 27 CYP3A4 protein variants to metabolize regorafenib in vitro. Basic Clin Pharmacol Toxicol 125(4):337–344. https://doi.org/10.1111/bcpt.13246
Ohya H, Shibayama Y, Ogura J, Narumi K, Kobayashi M, Iseki K (2015) Regorafenib is transported by the organic anion transporter 1B1 and the multidrug resistance protein 2. Biol Pharm Bull 38(4):582–586. https://doi.org/10.1248/bpb.b14-00740
Kort A, Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH (2015) Brain and Testis Accumulation of Regorafenib is Restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm Res 32(7):2205–2216. https://doi.org/10.1007/s11095-014-1609-7
Fujita KI, Masuo Y, Yamazaki E, Shibutani T, Kubota Y, Nakamichi N, Sasaki Y, Kato Y (2017) Involvement of the Transporters P-Glycoprotein and Breast Cancer Resistance Protein in Dermal Distribution of the Multikinase Inhibitor Regorafenib and Its Active Metabolites. J Pharm Sci 106(9):2632–2641. https://doi.org/10.1016/j.xphs.2017.04.064
Al-Shammari AH, Masuo Y, Fujita KI, Yoshikawa Y, Nakamichi N, Kubota Y, Sasaki Y, Kato Y (2019) Influx and Efflux Transporters Contribute to the Increased Dermal Exposure to Active Metabolite of Regorafenib After Repeated Oral Administration in Mice. J Pharm Sci 108(6):2173–2179. https://doi.org/10.1016/j.xphs.2019.01.018
Hafner FT, Werner D, Kaiser M (2014) Determination of regorafenib (BAY 73-4506) and its major human metabolites BAY 75-7495 (M-2) and BAY 81-8752 (M-5) in human plasma by stable-isotope dilution liquid chromatography-tandem mass spectrometry. Bioanalysis 6(14):1923–1937. https://doi.org/10.4155/bio.14.52
Luethi D, Durmus S, Schinkel AH, Schellens JH, Beijnen JH, Sparidans RW (2014) Liquid chromatography-tandem mass spectrometric assay for the multikinase inhibitor regorafenib in plasma. Biomed Chromatogr 28(10):1366–1370. https://doi.org/10.1002/bmc.3176
Kubota Y, Fujita KI, Takahashi T, Sunakawa Y, Ishida H, Hamada K, Ichikawa W, Tsunoda T, Shimada K, Masuo Y, Kato Y, Sasaki Y (2020) Higher Systemic Exposure to Unbound Active Metabolites of Regorafenib Is Associated With Short Progression-Free Survival in Colorectal Cancer Patients. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.1810
Sunakawa Y, Furuse J, Okusaka T, Ikeda M, Nagashima F, Ueno H, Mitsunaga S, Hashizume K, Ito Y, Sasaki Y (2014) Regorafenib in Japanese patients with solid tumors: phase I study of safety, efficacy, and pharmacokinetics. Investig New Drugs 32(1):104–112. https://doi.org/10.1007/s10637-013-9953-8
Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, Dockter TJ, Jacobs NL, Pasche BC, Cleary JM, Meyers JP, Desnoyers RJ, McCune JS, Pedersen K, Barzi A, Chiorean EG, Sloan J, Lacouture ME, Lenz HJ, Grothey A (2019) Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 20(8):1070–1082. https://doi.org/10.1016/s1470-2045(19)30272-4
Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP (2009) Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol 20(5):807–815. https://doi.org/10.1093/annonc/mdn713
Mukai M, Komori K, Oka T (2018) Mechanism and management of cancer chemotherapy-induced atherosclerosis. J Atheroscler Thromb 25(10):994–1002. https://doi.org/10.5551/jat.RV17027
Kawakami K, Wakatsuki T, Soejima A, Kobayashi K, Yokokawa T, Aoyama T, Suzuki K, Suenaga M, Yamaguchi K, Inoue A, Machida Y, Hama T (2019) Factors associated with regorafenib adherence with metastatic colorectal cancer. Patient Prefer Adherence 13:1745–1750. https://doi.org/10.2147/ppa.S217835
Campbell SD, de Morais SM, Xu JJ (2004) Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact 150(2):179–187. https://doi.org/10.1016/j.cbi.2004.08.008
Mross K, Frost A, Steinbild S, Hedbom S, Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O, Christensen O (2012) A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 18(9):2658–2667. https://doi.org/10.1158/1078-0432.CCR-11-1900
Wakatsuki T, Suenaga M, Shinozaki E, Nagayama S, Nakayama I, Matsushima T, Ogura M, Ichimura T, Takahari D, Chin K, Kumekawa Y, Sato Y, Fukunaga Y, Ueno M, Mizunuma N, Yamaguchi T (2015) 2175 Genetic variants of UGT1A1 and 1A9 could be associated with Regorafenib induced toxicity in Japanese patients with metastatic colorectal cancer. European Journal of Cancer 51:S391–S392. https://doi.org/10.1016/S0959-8049(16)31095-4
Miura Y, Imamura CK, Fukunaga K, Katsuyama Y, Suyama K, Okaneya T, Mushiroda T, Ando Y, Takano T, Tanigawara Y (2014) Sunitinib-induced severe toxicities in a Japanese patient with the ABCG2 421 AA genotype. BMC Cancer 14:964. https://doi.org/10.1186/1471-2407-14-964
Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H, Sugiyama Y (2006) Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther 79(5):427–439. https://doi.org/10.1016/j.clpt.2006.01.011
Saito Y, Sai K, Maekawa K, Kaniwa N, Shirao K, Hamaguchi T, Yamamoto N, Kunitoh H, Ohe Y, Yamada Y, Tamura T, Yoshida T, Minami H, Ohtsu A, Matsumura Y, Saijo N, Sawada J (2009) Close association of UGT1A9 IVS1+399C>T with UGT1A1*28, *6, or *60 haplotype and its apparent influence on 7-ethyl-10-hydroxycamptothecin (SN-38) glucuronidation in Japanese. Drug Metab Dispos 37(2):272–276. https://doi.org/10.1124/dmd.108.024208
Acknowledgements
First, we thank all the patients who participated in this study. Furthermore, we would like to thank Chiyo Imamura for assistance during the clinical study.
Funding
This work was supported by Japan Research Foundation for Clinical Pharmacology (to Dr. Wakatsuki).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Yamaguchi has received speaking honorariums from Taiho Pharmaceutical, Chugai Pharmaceutical, Merck Serono, Takeda Pharmaceutical, Yakult, Bayer, Ono Pharmaceutical, Eli Lilly, Sanofi and Bristol-Myers Squibb, and has received research grants from MSD, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Taiho Pharmaceutical, Daiichi Sankyo, Eli Lilly, Gilead Sciences, and Yakult. Also, he has served in a consulting or advisory role for Bristol-Myers Squibb. The other authors declare that they have no conflicts of interest.
Ethical approval
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and independently reviewed and approved by the Clinical Research Ethics Review Committee of the hospital (approval no. 2017-1205). All patients provided written informed consent for the use of their blood samples and medical information for research purposes. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN no. 000032725).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, K., Sugiyama, E., Shinozaki, E. et al. Associations among plasma concentrations of regorafenib and its metabolites, adverse events, and ABCG2 polymorphisms in patients with metastatic colorectal cancers. Cancer Chemother Pharmacol 87, 767–777 (2021). https://doi.org/10.1007/s00280-021-04237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04237-x