Abstract
Purpose
Immune checkpoint inhibitor-associated interstitial lung disease (ICI-ILD) is a serious immune-related adverse event. We aimed to evaluate the impact of ICI-ILD severity and imaging patterns or post-ILD cancer therapy on prognosis in patients with non-small-cell lung cancer (NSCLC).
Methods
We retrospectively analysed NSCLC patients who developed ICI-ILD in our institution between January 2016 and March 2019. The primary objective was to report prognosis following onset of ICI-ILD, stratified by severity grade or imaging pattern. The secondary objective was the analysis of cancer therapy after ICI-ILD.
Results
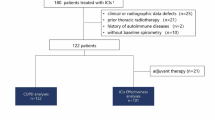
Among 222 patients treated with ICI, 27 (12.2%) developed ICI-ILD. No trend for different prognosis depending on severity grade was seen unless ICI-ILD was fatal. Most patients (91.3%) with organising pneumonia (OP) or nonspecific interstitial pneumonia pattern on imaging showed grade 1 or 2, while all patients with a diffuse alveolar damage (DAD) pattern showed grade 3 or higher, and one reached grade 5. Among patients who overcame ICI-ILD, eight patients (30.8%) have been followed up without chemotherapy because of long-term disease control and seven had shown an OP pattern on imaging at onset of ICI-ILD. Three patients underwent ICI rechallenge, but two showed ICI-ILD recurrence and no patient achieved response to rechallenge treatment.
Conclusion
The DAD pattern may predict short-term adverse prognosis for ICI-ILD. Once ICI-ILD is overcome, severity grade is not associated with prognosis. Even if initial immunotherapy proves effective, ICI rechallenge requires careful consideration.


Similar content being viewed by others
Data availability
Research data are not shared.
References
Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O (2016) Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54:139–148
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391
Naidoo J, Wang X, Woo KM, Lyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD (2017) Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35:709–717
Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N, Guisier F, Planchard D, Metivier AC, Tomasini P, Dansin E, Pérol M, Campana M, Gautschi O, Früh M, Fumet JD, Audigier-Valette C, Couraud S, Dalle S, Leccia MT, Jaffro M, Collot S, Prévot G, Milia J, Mazieres J (2017) Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 50:1700050
Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS (2016) Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2:1607–1616
Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning YM, Singh H, Suzman D, Xu J, Goldberg KB, Sridhara R, Ibrahim A, Theoret M, Beaver JA, Pazdur R (2019) Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 37:2730–2737
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378
Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS (2016) PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 22:6051
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, ATS/ERS Committee on Idiopathic Interstitial Pneumonias (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 188:733–748
Gobbini E, Charles J, Toffart AC, Leccia MT, Moro-Sibilot D, Levra MG (2019) Current opinions in immune checkpoint inhibitors rechallenge in solid cancers. Crit Rev Oncol Hematol 144:102816
Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF, Legallois D, Milliez PU, Lelong-Boulouard V, Alexandre J (2020) Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 6:865–871
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 5:649–655
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D (2009) Verweij J New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247
Suzuki Y, Karayama M, Uto T, Fujii M, Matsui T, Asada K, Kusagaya H, Kato M, Matsuda H, Matsuura S, Toyoshima M, Mori K, Ito Y, Koyauchi T, Yasui H, Hozumi H, Furuhashi K, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Suda T (2020) Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: a multicenter prospective study. J Thorac Oncol 15:1317–1327
Bernchou U, Schytte T, Bertelsen A, Bentzen SM, Hansen O, Brink C (2013) Time evolution of regional CT density changes in normal lung after IMRT for NSCLC. Radiother Oncol. 109:89–94
Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, Cho YJ, Yoon HI, Lee JH, Lee CT, Park JS (2018) Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 125:150–156
Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, Brahmer JR, Feller-Kopman D, Lerner AD, Lee H, Yarmus L, D’Alessio F, Hales RK, Lin CT, Psoter KJ, Danoff SK, Naidoo J (2018) Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 13:1930–1939
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, Han C, Long Y, Li Y, Zheng X, Sun D, Zhang J, Cai S, Jiao S, Hu Y (2018) Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med 7:4115–4120
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, Japan Thoracic Radiology Group, Jiang H, Itoh Y, Armour A, Watkins C, Higenbottam T, Nyberg F (2008) Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 177:1348–1357
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Suzuki K, Yanagihara T, Matsumoto K, Kusaba H, Yamauchi T, Ikematsu Y, Tanaka K, Otsubo K, Inoue H, Yoneshima Y, Iwama E, Arimura-Omori M, Harada E, Hamada N, Okamoto I, Nakanishi Y (2020) Immune-checkpoint profiles for T cells in bronchoalveolar lavage fluid of patients with immune-checkpoint inhibitor-related interstitial lung disease. Int Immunol 32:547–557
Meyer KC, Raghu G, Braughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C, Wood B, American Thoracic Society Committee on BAL in Interstitial Lung Disease (2012) American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 185:1004–1014
Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, Rouche JA, Zitvogel L, Zalcman G, Albiges L, Escudier B, Routy B (2018) Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 29:1437–1444
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, Sabari JK, Caramella C, Plodkowski AJ, Halpenny D, Chaft JE, Planchard D, Riely GJ, Besse B, Hellmann MD (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36:2872–2878
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Kaffenberger BH, Lunning M, McGettingan S, McPherson J, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Pannell N, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wang Y, Weight RM, Johnson-Chilla A, Zuccarino-Catania G, Engh A (2020) NCCN guidelines insights: management of Immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw 18:230–241
Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, Hisakane K, Takahashi S, Tanaka T, Takeuchi S, Miyanaga A, Minegishi Y, Noro R, Kubota K, Gemma A (2020) Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer 11:1052–1060
Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A, Michot JM, Postel-Vinay S, Angevin E, Ribrag V, Hollebecque A, Soria JC, Robert C, Massard C, Marabelle A (2019) Long-term survival in patients responding to Anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin Cancer Res 25:946–956
Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, Brahmer JR, Feller-Kopman D, Lerner AD, Lee H, Yarmus L, Hales RK, D’Alessio F, Danoff SK, Naidoo J (2019) Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol 14:494–502
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, Kris MG, Rudin CM, Chaft JE, Hellmann MD (2018) Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6:1093–1099
Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Laparra A, Vozy A, Varga A, Hollebecque A, Champiat S, Marabelle A, Massard C, Lambotte O (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5:1310–1317
Acknowledgments
The authors would like to thank all their colleagues who recruited and treated patients with ICI-ILD in this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AY served as the principal author, had full access to all data in the study, and takes responsibility for the integrity and accuracy of the data and data analysis. AY, TY and YF contributed to study conception and design; AI, TY and YF contributed to acquisition of data; AY and TY contributed to analysis and interpretation of data; AY, TY, YF and TI contributed to drafting and revision of the manuscript and approved the final version submitted for consideration for publication.
Corresponding author
Ethics declarations
Conflict of interest
TI has received honoraria from MSD K.K. The other authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the institutional review board at Kurashiki Central Hospital (approval no. 3108).
Consent to participate
The requirement for consent to participate was waived because of the retrospective design of the study.
Consent for publication
The requirement for consent to publication was waived because of the retrospective design of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamagata, A., Yokoyama, T., Fukuda, Y. et al. Impact of interstitial lung disease associated with immune checkpoint inhibitors on prognosis in patients with non-small-cell lung cancer. Cancer Chemother Pharmacol 87, 251–258 (2021). https://doi.org/10.1007/s00280-020-04205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04205-x