Abstract
Purpose
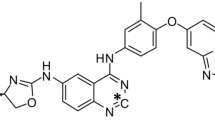
H3B-6545, a novel selective estrogen receptor (ER)α covalent antagonist (SERCA) which inactivates both wild-type and mutant ERα, is in clinical development for the treatment of metastatic breast cancer. Preclinical studies were conducted to characterize the pharmacokinetics and metabolism of H3B-6545 in rat and monkeys.
Methods
The clearance and metabolic profiles of H3B-6545 were studied using rat, monkey and human hepatocytes, and reaction phenotyping was done using recombinant human cytochrome P450 enzymes. Blood stability, protein binding, and permeability were also determined in vitro. Pharmacokinetics of H3B-6545 was assessed after both intravenous and oral dosing. A nonclinical PBPK model was developed to assess in vitro–in vivo correlation of clearance.
Results
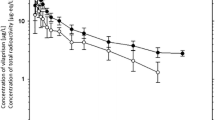
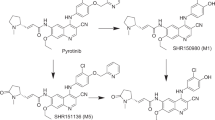
H3B-6545 had a terminal elimination half-life of 2.4 h in rats and 4.0 h in monkeys and showed low to moderate bioavailability, in line with the in vitro permeability assessment. Plasma protein binding was similar across species, at 99.5–99.8%. Nine metabolites of H3B-6545 were identified in hepatocyte incubations, none of which were unique to humans. Formation of glutathione-related conjugate of H3B-6545 was minimal in vitro. H3B-6545, a CYP3A substrate, is expected to be mostly cleared via hepatic phase 1 metabolism. Hepatocyte clearance values were used to adequately model the time-concentration profiles in rat and monkey.
Conclusions
We report on the absorption and metabolic fate and disposition of H3B-6545 in rats and dogs and illustrate that in vitro–in vivo correlation of clearance is possible for targeted covalent inhibitors, provided reactivity is not a predominant mechanism of clearance.




Similar content being viewed by others
References
Kauhava L, Immonen-Räihä P, Parvinen I, Holli K, Pylkkänen L, Kaljonen A, Helenius H, Kronqvist P, Klemi PJ (2008) Lower recurrence risk through mammographic screening reduces breast cancer treatment costs. Breast 17:550–554
Spicer DV, Pike MC (1993) Breast cancer prevention through modulation of endogenous hormones. Breast Cancer Res Treat 28:179–193
Beslija S, Bonneterre J, Burstein HJ et al (2009) Third consensus on medical treatment of metastatic breast cancer. Ann Oncol 20:1771–1785
Chandarlapaty S, Chen D, He W et al (2016) Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol 2:1310–1315
Puyang X, Furman C, Zheng GZ et al (2018) Discovery of selective estrogen receptor covalent antagonists for the treatment of ERαWT and ERαMUT breast cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-17-1229
Leung L, Yang X, Strelevitz TJ, Montgomery J, Brown MF, Zientek MA, Banfield C, Gilbert AM, Thorarensen A, Dowty ME (2017) Clearance prediction of targeted covalent inhibitors by in vitro–in vivo extrapolation of hepatic and extrahepatic clearance mechanisms. Drug Metab Dispos 45:1–7
Colombo F, Smith S, Korpal M, Hao MH, Nix D, O’Shea M, Prajapati S, Wang J, Warmuth M, Smith PG, Rioux N (2018) In vitro-in vito correlation of clearance for H3B-5942, a novel selective ERα covalent antagonist (SERCA). In: 22nd North American ISSX meeting, abstract P10. https://bib.irb.hr/datoteka/947954.22nd_north_american_issx_meeting_abstract_book.pdf
Smith PG, Puyang X, Furman C et al (2017) Discovery and development of H3B-6545: a novel, oral, selective estrogen receptor covalent antagonist (SERCA) for the treatment of breast cancer. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017, AACR, Washington DC. Cancer Res 77(13 Suppl):Abstract nr DDT01-04
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Pollack GM, Brouwer KLR, Demby KB, Jones JA (1990) Determination of hepatic blood flow in the rat using sequential infusions of indocyanine green and galactose. Drug Metab Dispos 18:197–202
Nakajima M, Nakamura S, Tokudome S, Shimada N, Yamazaki H, Yokoi T (1999) Azelastine N-demethylation by cytochrome P450 (CYP)3A4, CYP2D6, and CYP1A2 in human liver microsomes: evaluation of approach to predict the contribution of multiple CYPs. Drug Metab Dispos 27:1381–1391
Drug development and drug interactions: table of substrates, inhibitors and inducers. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm Accessed 2 July 2018
Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, Leggas M, Ejendal KF, Hrycyna CA, Schuetz JD (2007) Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res 67:6965–6972
Gillette JR (1971) Factors affecting drug metabolism. Ann NY Acad Sci 179:43–66
Rowland M, Benet LZ, Graham GG (1973) Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm 1:123–136
Obach RS (1999) Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos 27:1350–1359
Mackenzie PI, Rogers A, Treloar J, Jorgensen BR, Miners JO, Meech R (2008) Identification of UDP glycosyltransferase 3A1 as a UDP N-acetylglucosaminyltransferase. J Biol Chem 283:36205–36210
Stopfer P, Marzin K, Narjes H, Gansser D, Shahidi M, Uttereuther-Fischer M, Ebner T (2012) Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 69:1051–1061
Scheers E, Leclercq L, de Jong J, Bode N, Bockx M, Laenen A, Cuyckens F, Skee D, Murphy J, Sukbuntherng J, Mannens G (2015) Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab Dispos 43:289–297
Baillie TA (2016) Targeted covalent inhibitors for drug design. Angew Chem Int Ed 55:13408–13421
Shibata Y, Chiba M (2015) The role of extrahepatic metabolism in the pharmacokinetics of the targeted covalent inhibitors afatinib, ibrutinib, and neratinib. Drug Metab Dispos 43:375–384
Commandeur JN, Stijntjes GJ, Vermeulen NP (1995) Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role of bioactivation and detoxification mechanisms of xenobiotics. Pharmacol Rev 47:271–330
Dickinson PA, Cantarini MV, Collier J, Frewer P, Martin S, Pickup K, Ballard P (2016) Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos 44:1201–1212
Block M (2015) Physiologically based pharmacokinetic and pharmacodynamic modeling in cancer drug development: status, potential and gaps. Expert Opin Drug Metab Toxicol 11:743–756
Acknowledgements
The authors want to thank the H3B-6545 team at H3 Biomedicine for fruitful discussions.
Funding
This study was funded by H3 Biomedicine Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and/or animal participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Rioux, N., Smith, S., Korpal, M. et al. Nonclinical pharmacokinetics and in vitro metabolism of H3B-6545, a novel selective ERα covalent antagonist (SERCA). Cancer Chemother Pharmacol 83, 151–160 (2019). https://doi.org/10.1007/s00280-018-3716-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3716-3