Abstract
Purpose
Epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR–TKI) is effective as first-line chemotherapy for patients with advanced non-small-cell lung cancer (NSCLC) harboring sensitive EGFR mutations. However, whether the efficacy of second-line cytotoxic drug chemotherapy after first-line EGFR–TKI treatment is similar to that of first-line cytotoxic drug chemotherapy in elderly patients aged ≥ 75 years harboring sensitive EGFR mutations is unclear. Therefore, we aimed to investigate the efficacy and safety of cytotoxic drug chemotherapy after first-line EGFR–TKI treatment in elderly patients with NSCLC harboring sensitive EGFR mutations.
Methods
We retrospectively evaluated the clinical effects and safety profiles of second-line cytotoxic drug chemotherapy after first-line EGFR–TKI treatment in elderly patients with NSCLC harboring sensitive EGFR mutations (exon 19 deletion/exon 21 L858R mutation). Between April 2008 and December 2015, 78 elderly patients with advanced NSCLC harboring sensitive EGFR mutations received first-line EGFR–TKI at four Japanese institutions. Baseline characteristics, regimens, responses to first- and second-line treatments, whether or not patients received subsequent treatment, and if not, the reasons for non-administration were recorded.
Results
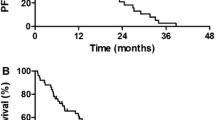
Overall, 20 patients with a median age of 79.5 years (range 75–85 years) were included in our analysis. The overall response, disease control, median progression-free survival, and overall survival rates were 15.0, 60.0%, 2.4, and 13.2 months, respectively. Common adverse events included leukopenia, neutropenia, anemia, thrombocytopenia, malaise, and anorexia. Major grade 3 or 4 toxicities included leukopenia (25.0%) and neutropenia (45.0%). No treatment-related deaths were noted.
Conclusion
Second-line cytotoxic drug chemotherapy after first-line EGFR–TKI treatment among elderly patients with NSCLC harboring sensitive EGFR mutations was effective and safe and showed equivalent outcomes to first-line cytotoxic drug chemotherapy.



Similar content being viewed by others
References
Miller KD, Siegel RL, Lin CC et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289
Davidoff AJ, Tang M, Seal B, Edelman MJ (2010) Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 28:2191–2197
Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, Ramalingam SS (2007) Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 25:5570–5577
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Rosell R, Carcereney E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Zhou C, Wu YL, Chen G et al (2015) Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 26:1877–1883
Yang JC, Wu YL, Schuler M et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16:141–151
Maemondo M, Minegishi Y, Inoue A et al (2012) First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 7:1417–1422
Fujita S, Katakami N, Masago K et al (2012) Customized chemotherapy based on epidermal growth factor receptor mutation status for elderly patients with advanced non-small-cell lung cancer: a phase II trial. BMC Cancer 12:185
Tateishi K, Ichiyama T, Hirai K et al (2013) Clinical outcomes in elderly patients administered gefitinib as first-line treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer: retrospective analysis in a Nagano Lung Cancer Research Group study. Med Oncol 30:450
Goto K, Nishio M, Yamamoto N et al (2013) A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 82:109–114
Takahashi K, Saito H, Hashegawa Y et al (2014) First-line gefitinib therapy for elderly patients with non-small cell lung cancer harboring EGFR mutation: Central Japan Lung Study Group 0901. Cancer Chemother Pharmacol 74:721–727
Kuwako T, Imai H, Masuda T et al (2015) First-line gefitinib treatment in elderly patients (aged>/=75 years) with non-small cell lung cancer harboring EGFR mutations. Cancer Chemother Pharmacol 76:761–769
Yoshino R, Imai H, Mori K et al (2014) Surrogate endpoints for overall survival in advanced non-small-cell lung cancer patients with mutations of the epidermal growth factor receptor gene. Mol Clin Oncol 2:731–736
Inoue A, Kobayashi K, Maemondo M et al (2013) Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24:54–59
Kobayashi S, Boggon TJ, Dayaram T et al (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352:786–792
Sequist LV, Waltman BA, Dias-Santagata D et al (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3:75ra26
Oxnard GR, Arcila ME, Sima CS et al (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17:1616–1622
Yu HA, Arcila ME, Rekhtman N et al (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19:2240–2247
Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W (2011) New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res 17:5530–5537
Gridelli C, Shepherd FA (2005) Chemotherapy for elderly patients with non-small cell lung cancer: a review of the evidence. Chest 128:947–957
Belani CP, Fossella F (2005) Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326). Cancer 104:2766–2774
Novello S, Barlesi F, Califano R et al (2016) Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v1-v27
No authors listed (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 91:66–72
Gridelli C, Perrone F, Gallo C et al (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Kudoh S, Takeda K, Nakagawa K et al (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663
Goldstraw P, Crowley J, Chansky K et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706–714
Nagai Y, Miyazawa H, Hugun et al (2005) Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res 65:7276–7282
Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn 8:335–341
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Abe T, Takeda K, Ohe Y et al (2015) Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol 33:575–581
Takeuchi S, Yano S (2014) Clinical significance of epidermal growth factor receptor tyrosine kinase inhibitors: sensitivity and resistance. Respir Investig 52:348–356
Mok TS, Wu YL, Ahn MJ et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376:629–640
Soria JC, Wu YL, Nakagawa Y et al (2015) Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 16:990–998
Quoix E, Zalcman G, Oster JP et al (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378:1079–1088
Acknowledgements
We thank Drs. Tomohiro Tamura, Sakae Fujimoto, Hiroshi Yokouchi, Yoichi Nakamura, and Takeshi Hisada for their assistance in preparing this manuscript. We also thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any financial or personal relationships with people or organizations that could inappropriately influence this work.
Ethical approval
The institutional review board of each institution approved the study protocol.
Informed consent
The need for informed consent was waived by the Institutional Review Boards of each participating institution because of the retrospective nature of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imai, H., Minemura, H., Sugiyama, T. et al. Efficacy and safety of cytotoxic drug chemotherapy after first-line EGFR–TKI treatment in elderly patients with non-small-cell lung cancer harboring sensitive EGFR mutations. Cancer Chemother Pharmacol 82, 119–127 (2018). https://doi.org/10.1007/s00280-018-3596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3596-6