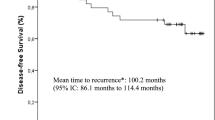
Abstract
Background
Pegylated liposomal doxorubicin (PLD) is used as a second-line therapy for gynecologic cancers, with a better short-term toxicity profile compared to doxorubicin or other anthracyclines.
Methods
We screened 14 patients with recurrent gynecologic cancers, who underwent prolonged treatment with large cumulative doses of PLD for overt or subtle signs of cardiotoxicity (CTX) using standard and advanced echocardiography techniques [3D volumetric method for left ventricular ejection fraction (LVEF) and left ventricular/right ventricular global longitudinal strain]. Half the patients had previous echocardiographic studies available for comparison.
Results
The average PLD treatment duration was 23.6 ± 10.8 months (range 13–57), accumulating dose of 1387 ± 483 mg (range 780–2538 mg). The study group had a normal LVEF both by 2D-echo (60 ± 5%, range 50–67) and 3D echo (58 ± 5%, range 46–63). Two patients (14%) were found to have minimally reduced ejection fraction by 2D and 3D echo (50%/46% and 51%/49%, respectively) that did not meet the current definition of CTX. For the seven patients who had consecutive echocardiography studies, the average LVEF remained stable between studies (59 ± 7, 60 ± 9 and 58 ± 10.5% for the latest study, previous, p < 0.79, and most remote study p < 0.9); No change was found in average left ventricular/right ventricular global longitudinal strain as well: −20.8 ± 4.6% at the latest study and −19.3 ± 2.6% for the previous (p < 0.51).
Conclusion
No prevalent or incident cases of cardiotoxicity were found despite prolonged treatment with large cumulative doses of PLD, adding to previous reports on shorter treatment duration.


Similar content being viewed by others
References
Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB (2008) Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 26(22):3777–3784
Schwartz RG, Jain D, Storozynsky E (2013) Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol 20(3):443–464
Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA (1973) A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32(2):302–314
Sheppard RJ, Berger J, Sebag IA (2013) Cardiotoxicity of cancer therapeutics: current issues in screening, prevention, and therapy. Front Pharmacol 4:19
Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91(5):710–717
Colombo A, Cipolla C, Beggiato M, Cardinale D (2013) Cardiac toxicity of anticancer agents. Curr Cardiol Rep 15(5):362
Gabizon A, Shmeeda H, Grenader T (2012) Pharmacological basis of pegylated liposomal doxorubicin: impact on cancer therapy. Eur J Pharm Sci 45(4):388–398
Staropoli N, Ciliberto D, Botta C, Fiorillo L, Grimaldi A, Lama S, Caraglia M, Salvino A, Tassone P, Tagliaferri P (2014) Pegylated liposomal doxorubicin in the management of ovarian cancer: a systematic review and metaanalysis of randomized trials. Cancer Biol Ther 15(6):707–720
Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J (2013) Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev 7:CD006910
Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A (2000) Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 11(8):1029–1033
Orditura M, Quaglia F, Morgillo F, Martinelli E, Lieto E, De Rosa G, Comunale D, Diadema MR, Ciardiello F, Catalano G et al (2004) Pegylated liposomal doxorubicin: pharmacologic and clinical evidence of potent antitumor activity with reduced anthracycline-induced cardiotoxicity (review). Oncol Rep 12(3):549–556
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP et al (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 27(9):911–939
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270
Gill SE, Savage K, Wysham WZ, Blackhurst DW, Winter WE, Puls LE (2013) Continuing routine cardiac surveillance in long-term use of pegylated liposomal doxorubicin: is it necessary? Gynecol Oncol 129(3):544–547
Yildirim Y, Gultekin E, Avci ME, Inal MM, Yunus S, Tinar S (2008) Cardiac safety profile of pegylated liposomal doxorubicin reaching or exceeding lifetime cumulative doses of 550 mg/m2 in patients with recurrent ovarian and peritoneal cancer. Int J Gynecol Cancer 18(2):223–227
Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P (2009) Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr 10(2):194–212
Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP et al (2012) EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 25(1):3–46
Feigenbaum H, Mastouri R, Sawada S (2012) A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J 76(7):1550–1555
D’Hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR (2000) Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 1(3):154–170
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP et al (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 107(9):1375–1380
Gorcsan J 3rd, Tanaka H (2011) Echocardiographic assessment of myocardial strain. J Am Coll Cardiol 58(14):1401–1413
Hedhli N, Russell KS (2011) Cardiotoxicity of molecularly targeted agents. Curr Cardiol Rev 7(4):221–233
Acknowledgements
This work was made possible in part by Grants from the Montreal-Israel Cancer Research Foundation, the Gloria Shapiro fund, the Hospital Foundation, and the Levy Family Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Blank, N., Laskov, I., Kessous, R. et al. Absence of cardiotoxicity with prolonged treatment and large accumulating doses of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol 80, 737–743 (2017). https://doi.org/10.1007/s00280-017-3412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3412-8