Abstract
Purpose
To characterize the cardiovascular safety profile of regorafenib in patients with advanced cancer.
Methods
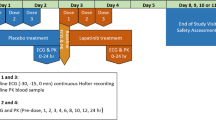
Patients received regorafenib 160 mg/day for 21 days followed by a 7-day break. The primary endpoint was the change from baseline in QTcF at the regorafenib t max (Day 21, Cycle 1 or 2) and changes in left ventricular ejection fraction (LVEF) from baseline on Cycle 2, Day 21. Secondary objectives were pharmacokinetics, safety, anti-tumor activity and effects on electrocardiogram intervals. QT intervals were corrected using the methods of Fridericia (QTcF) and Bazett (QTcB). LVEF was assessed by multigated acquisition scanning.
Results
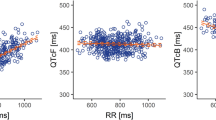
Fifty-three patients were enrolled, and all received at least one dose of regorafenib 160 mg. Twenty-five patients received regorafenib for 21 days without dose reduction. The mean change from baseline in QTcF at t max was (−)2 ms (90 % CI −8, 3). No patient experienced a change from baseline in QTcF > 60 ms, and two had QTcF changes between 30 and 60 ms. No patient had a QTcF or QTcB > 480 ms. In 27 patients who received at least 80 mg of regorafenib, the mean change from baseline in LVEF% ± SD was 1.7 ± 7.8. In 14 patients without a dose reduction, the mean change from baseline in LVEF% was (−)0.1 ± 8.6 at Cycle 2, Day 21. Four patients experienced a LVEF decrease between 10 and 20 %.
Conclusion
The effects of regorafenib on the QT/QTc interval and LVEF were modest and unlikely to be of clinical significance in the setting of advanced cancer therapy.


Similar content being viewed by others
References
Wilhelm SM, Dumas J, Adnane L et al (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–255
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Fabian MA, Biggs WH 3rd, Treiber DK et al (2005) A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23:329–336
International Conference on Harmonisation (2005) Guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Fed Regist 70:61134-61135
Strumberg D, Scheulen ME, Schultheis B et al (2012) Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer 106:1722–1727
Mross K, Frost A, Steinbild S et al (2012) A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 18:2658–2667
Khakoo AY, Kassiotis CM, Tannir N et al (2008) Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer 112:2500–2508
Machiels JP, Bletard N, Pirenne P, Jacquet L, Bonbled F, Duck L (2008) Acute cardiac failure after sunitinib. Ann Oncol 19:597–599
Schmidinger M, Zielinski CC, Vogl UM et al (2008) Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 26:5204–5212
Chu TF, Rupnick MA, Kerkela R et al (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370:2011–2019
Telli ML, Witteles RM, Fisher GA, Srinivas S (2008) Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 19:1613–1618
Di Lorenzo G, Autorino R, Bruni G et al (2009) Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol 20:1535–1542
Tolcher AW, Appleman LJ, Shapiro GI et al (2011) A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol 67:751–764
Heath EI, Infante J, Lewis LD et al (2013) A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors. Cancer Chemother Pharmacol 71:565–573
Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK (2014) QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer 112:296–305
Acknowledgments
This trial was sponsored by Bayer HealthCare Pharmaceuticals. We acknowledge the assistance of Ann Contijoch, Bayer Healthcare Pharmaceuticals, in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.L. Jones serves as an advisory board member for Immune Design, Daiichi Sankyo, Merck, and Blueprint Medicines. He also reports conducting trials sponsored by Bayer HealthCare Pharmaceuticals, Eisai, Novartis, Johnson & Johnson, Pharmamar, Infinity Pharmaceuticals, Threshold Pharmaceuticals, and Morphotek. D.C. Smith has received research funding from Bayer HealthCare Pharmaceuticals. K. Diefenbach, J. Lettier, and O. Boix are employees of Bayer HealthCare Pharmaceuticals; K. Diefenbach and O Boix also report stock ownership in Bayer HealthCare Pharmaceuticals. A.C. Lockhart has received research funding from Amgen, Bayer, Daiichi Sankyo, EMD Serono, Genentech/Roche, Lilly, Millennium Takeda, Novartis, Sanofi, Teva, and Zenyaku Kogyo. K.N. Moore serves as an advisory board member for Astrazeneca, Immunogen, Genentech, Amgen, and Advaxis. J.C. Bendell and C.L. O'Bryant report no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jones, R.L., Bendell, J.C., Smith, D.C. et al. A phase I open-label trial evaluating the cardiovascular safety of regorafenib in patients with advanced cancer. Cancer Chemother Pharmacol 76, 777–784 (2015). https://doi.org/10.1007/s00280-015-2827-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2827-3