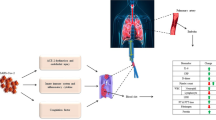
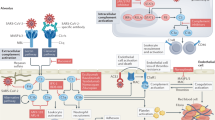
Abstract
The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), formerly known as 2019-nCoV. Numerous cellular and biochemical issues arise after COVID-19 infection. The severe inflammation that is caused by a number of cytokines appears to be one of the key hallmarks of COVID-19. Additionally, people with severe COVID-19 have coagulopathy and fulminant thrombotic events. We briefly reviewed the COVID-19 disease at the beginning of this paper. The inflammation and coagulation markers and their alterations in COVID-19 illness are briefly discussed in the parts that follow. Next, we talked about NETosis, which is a crucial relationship between coagulation and inflammation. In the end, we mentioned the two-way relationship between inflammation and coagulation, as well as the factors involved in it. We suggest that inflammation and coagulation are integrated systems in COVID-19 that act on each other in such a way that not only inflammation can activate coagulation but also coagulation can activate inflammation.
Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Wang L, Wang Y, Ye D, Liu Q (2020) Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents 55(6):105948
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109(6):1088–1095
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama 323(13):1239–1242
Aktas G (2020) A comprehensive review on rational and effective treatment strategies against an invisible enemy; SARS Cov-2 infection. Exp Biomed Res 3(4):293–311
Aktas G, Balci B, Yilmaz S, Bardak H, Duman TT, Civil C (2022) Characteristics of Covid-19 infection with the original SARS-Cov-2 virus and other variants: a comparative review. J Bionic Mem 2(3):96–112
Wong RSY (2021) Inflammation in COVID-19: from pathogenesis to treatment. Int J Clin Exp Pathol 14(7):831–844
Khalid A, Ali Jaffar M, Khan T, Abbas Lail R, Ali S, Aktas G et al (2021) Hematological and biochemical parameters as diagnostic and prognostic markers in SARS-COV-2 infected patients of Pakistan: a retrospective comparative analysis. Hematology 26(1):529–542
Aktas G (2021) Hematological predictors of novel coronavirus infection. Rev Assoc Med Bras 67:1–2
Fei Y, Tang N, Liu H, Cao W (2020) Coagulation dysfunctiona hallmark in COVID-19. Arch Pathol Lab Med 144(10):1223–1229
Hadid T, Kafri Z, Al-Katib A (2021) Coagulation and anticoagulation in COVID-19. Blood Rev 47:100761
Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T (2020) Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci 57(6):389–399
Becker RC (2020) Toward understanding the 2019 coronavirus and its impact on the heart. J Thromb Thrombolysis 50(1):33–42
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 183(6):1735
Lei J, Kusov Y, Hilgenfeld R (2018) Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antivir Res 149:58–74
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273
Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16(11):676–689
Cayrol C, Girard JP (2018) Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 281(1):154–168
Liang Y, Ge Y, Sun J (2021) IL-33 in COVID-19: friend or foe? Cell Mol Immunol 18(6):1602–1604
Gabryelska A, Kuna P, Antczak A, Białasiewicz P, Panek M (2019) IL-33 Mediated inflammation in chronic respiratory diseases-understanding the role of the member of IL-1 superfamily. Front Immunol 10:692
Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD et al (2020) Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med 8(8):750–752
Hayakawa H, Hayakawa M, Kume A, Tominaga S (2007) Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 282(36):26369–26380
Griesenauer B, Paczesny S (2017) The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 8:475
Zeng Z, Hong X-Y, Zhou H, Liao F-L, Guo S, Li Y et al (2020) Serum soluble ST2 as a novel biomarker reflecting inflammatory status and disease severity in patients with COVID-19. Available at SSRN 3594550
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T et al (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 5(7):811–818
Zeng Z, Hong XY, Li Y, Chen W, Ye G, Li Y, Luo Y (2020) Serum-soluble ST2 as a novel biomarker reflecting inflammatory status and illness severity in patients with COVID-19. Biomark Med 14(17):1619–1629
Baggiolini M, Walz A, Kunkel SL (1989) Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 84(4):1045–1049
Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M (1991) Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med 173(3):771–774
Ma A, Zhang L, Ye X, Chen J, Yu J, Zhuang L et al (2021) High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front Immunol 12:626235
Kang S, Tanaka T, Inoue H, Ono C, Hashimoto S, Kioi Y et al (2020) IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 117(36):22351–22356
Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA et al (2020) Neutrophil extracellular traps in COVID-19. JCI Insight 5(11).
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3):499–511
Kushner I (1982) The phenomenon of the acute phase response. Ann N Y Acad Sci 389:39–48
Ridker PM (2003) Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107(3):363–369
Kushner I, Jiang SL, Zhang D, Lozanski G, Samols D (1995) Do post-transcriptional mechanisms participate in induction of C-reactive protein and serum amyloid A by IL-6 and IL-1? Ann N Y Acad Sci 762:102–107
Calabró P, Willerson JT, Yeh ET (2003) Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation 108(16):1930–1932
Black S, Kushner I, Samols D (2004) C-reactive protein. J Biol Chem 279(47):48487–48490
Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, Berger JS (2021) C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J 42(23):2270–2279
Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B (2020) C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis 14:1753466620937175
Quinlan GJ, Martin GS, Evans TW (2005) Albumin: biochemical properties and therapeutic potential. Hepatology 41(6):1211–1219
Rothschild MA, Oratz M, Schreiber SS (1988) Serum albumin. Hepatology 8(2):385–401
Serum Albumin.
Paliogiannis P, Mangoni AA, Cangemi M, Fois AG, Carru C, Zinellu A (2021) Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): a systematic review and meta-analysis. Clin Exp Med 21(3):343–354
Bansal A, Prasad JB (2022) Liver profile in COVID-19: a meta-analysis. Z Gesundh Wiss 30(1):253–258
Parohan M, Yaghoubi S, Seraji A (2020) Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res 50(8):924–935
Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J et al (2020) Clinical characteristics of 82 cases of death from COVID-19. PLoS One 15(7):e0235458
Torun A, Çakırca TD, Çakırca G (1992) Portakal RD (2021) The value of C-reactive protein/albumin, fibrinogen/albumin, and neutrophil/lymphocyte ratios in predicting the severity of CoVID-19. Rev Assoc Med Bras 67(3):431–436
Park JE, Chung KS, Song JH, Kim SY, Kim EY, Jung JY et al (2018) The C-reactive protein/albumin ratio as a predictor of mortality in critically ill patients. J Clin Med 7(10):333
Kalabin A, Mani VR, Valdivieso SC, Donaldson B (2021) Does C reactive protein/albumin ratio have prognostic value in patients with COVID-19. J Infect Dev Ctries 15(08):1086–1093
Karagoz I, Ozer B, Ital I, Turkoglu M, Disikirik A, Ozer S (2023) C-reactive protein-to-serum albumin ratio as a marker of prognosis in adult intensive care population. Bratisl Lek Listy 124(4):277–279
Aktas G (2023) Serum C-reactive protein to albumin ratio as a reliable marker of diabetic neuropathy in type 2 diabetes mellitus. Preprints. https://doi.org/10.20944/preprints202306.0202.v1
Bilgin S, Kurtkulagi O, Tel BMA, Duman TT, Kahveci G, Khalid A, Aktas G (2021) Does C-reactive protein to serum albumin ratio correlate with diabetic nephropathy in patients with type 2 diabetes mellitus? The CARE TIME study. Prim Care Diabetes 15(6):1071–1074
Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H et al (2011) The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol 12:30
Kalyon S, Gültop F, Şimşek F, Adaş M (2021) Relationships of the neutrophil-lymphocyte and CRP-albumin ratios with the duration of hospitalization and fatality in geriatric patients with COVID-19. J Int Med Res 49(9):3000605211046112
Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K (1997) Procalcitonin--a new indicator of the systemic response to severe infections. Infection 25(6):329–334
Organization WH (2014) Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland, p 2017
Delèvaux I, André M, Colombier M, Albuisson E, Meylheuc F, Bègue RJ et al (2003) Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis 62(4):337–340
Russwurm S, Wiederhold M, Oberhoffer M, Stonans I, Zipfel PF, Reinhart K (1999) Molecular aspects and natural source of procalcitonin. Clin Chem Lab Med 37(8):789–797
Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS (2020) Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr 16(3):251–259
Chen X, Yang Y, Huang M, Liu L, Zhang X, Xu J et al (2020) Differences between COVID-19 and suspected then confirmed SARS-CoV-2-negative pneumonia: a retrospective study from a single center. J Med Virol 92(9):1572–1579
Yunus I, Fasih A, Wang Y (2018) The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS One 13(11):e0206527
Wolfisberg S, Gregoriano C, Schuetz P (2022) Procalcitonin for individualizing antibiotic treatment: an update with a focus on COVID-19. Crit Rev Clin Lab Sci 59(1):54–65
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368(16):1509–1518
Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M et al (2020) Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 146(1):128–36.e4
Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS (2020) IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev 53:13–24
Abbasifard M, Khorramdelazad H (2020) The bio-mission of interleukin-6 in the pathogenesis of COVID-19: a brief look at potential therapeutic tactics. Life Sci 257:118097
Potere N, Batticciotto A, Vecchié A, Porreca E, Cappelli A, Abbate A et al (2021) The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol 17(6):601–618
Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y et al (2020) Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 71(8):1937–1942
Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J et al (2020) The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med 12(7):e12421
Saraiva M, Vieira P, O'Garra A (2020) Biology and therapeutic potential of interleukin-10. J Exp Med 217(1):e20190418
Moore KW, de Waal MR, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765
Lu L, Zhang H, Dauphars DJ, He YW (2021) A potential role of interleukin 10 in COVID-19 Pathogenesis. Trends Immunol 42(1):3–5
Furlow B (2020) COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol 2(10):e592
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W et al (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9(1):1123–1130
Dhar SK, Vishnupriyan K, Damodar S, Gujar S, Das M (2021) IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon 7(2):e06155
Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J et al (2020) Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 5(13)
Weitz JI, Fredenburgh JC, Eikelboom JW (2017) A test in context: D-dimer. J Am Coll Cardiol 70(19):2411–2420
Adam SS, Key NS, Greenberg CS (2009) D-dimer antigen: current concepts and future prospects. Blood 113(13):2878–2887
Cohen AT, Spiro TE, Spyropoulos AC, Desanctis YH, Homering M, Büller HR et al (2014) D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost 12(4):479–487
Rao KM, Pieper CS, Currie MS, Cohen HJ (1994) Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol 102(6):802–805
Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS (2002) D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 121(4):1262–1268
Lowe GD, Yarnell JW, Rumley A, Bainton D, Sweetnam PM (2001) C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? Arterioscler Thromb Vasc Biol 21(4):603–610
Xu Y, Qian Y, Gu Q, Tang J (2020) Relationship between D-dimer concentration and inflammatory factors or organ function in patients with coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 32(5):559–563
Moreno G, Carbonell R, Bodí M, Rodríguez A (2021) Systematic review of the prognostic utility of D-dimer, disseminated intravascular coagulation, and anticoagulant therapy in COVID-19 critically ill patients. Med Intensiva (Engl Ed) 45(1):42–55
Weisel JW (2005) Fibrinogen and fibrin. Adv Protein Chem 70:247–299
Weisel JW, Litvinov RI (2017) Fibrin formation, structure and properties. Subcell Biochem 82:405–456
Levi M, Toh CH, Thachil J, Watson HG (2009) Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145(1):24–33
Han H, Yang L, Liu R, Liu F, Wu KL, Li J et al (2020) Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 58(7):1116–1120
Giannis D, Ziogas IA, Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 127:104362
Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M et al (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18(7):1747–1751
Morrissey JH, Fakhrai H, Edgington TS (1987) Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell 50(1):129–135
Morrissey JH (2001) Tissue factor: an enzyme cofactor and a true receptor. Thromb Haemost 86(1):66–74
Bauer W, Galtung N, Neuwinger N, Kaufner L, Langer E, Somasundaram R et al (2021) A Matter of caution: coagulation parameters in COVID-19 do not differ from patients with ruled-out SARS-CoV-2 infection in the emergency department. TH Open 5(1):e43–e55
Camerer E, Huang W, Coughlin SR (2000) Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A 97(10):5255–5260
Mackman N (2009) The many faces of tissue factor. J Thromb Haemost 7 Suppl 1(Suppl 1):136–139
Foley JH, Conway EM (2016) Cross talk pathways between coagulation and inflammation. Circ Res 118(9):1392–1408
Eslamifar Z, Behzadifard M, Soleimani M, Behzadifard S (2020) Coagulation abnormalities in SARS-CoV-2 infection: overexpression tissue factor. Thromb J 18(1):38
Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J et al (2003) Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 362(9400):1953–1958
Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR et al (2020) Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136(11):1330–1341
Rosell A, Havervall S, von Meijenfeldt F, Hisada Y, Aguilera K, Grover SP et al (2021) Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler Thromb Vasc Biol 41(2):878–882
Subrahmanian S, Borczuk A, Salvatore S, Fung KM, Merrill JT, Laurence J, Ahamed J (2021) Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J Thromb Haemost 19(9):2268–2274
Hassan MI, Saxena A, Ahmad F (2012) Structure and function of von Willebrand factor. Blood Coagul Fibrinolysis 23(1):11–22
Li S, Wang Z, Liao Y, Zhang W, Shi Q, Yan R et al (2010) The glycoprotein Ibalpha-von Willebrand factor interaction induces platelet apoptosis. J Thromb Haemost 8(2):341–350
Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA (2009) Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 324(5932):1330–1334
Bockmeyer CL, Claus RA, Budde U, Kentouche K, Schneppenheim R, Lösche W et al (2008) Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 93(1):137–140
Sporn LA, Chavin SI, Marder VJ, Wagner DD (1985) Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest 76(3):1102–1106
van Breevoort D, van Agtmaal EL, Dragt BS, Gebbinck JK, Dienava-Verdoold I, Kragt A et al (2012) Proteomic screen identifies IGFBP7 as a novel component of endothelial cell-specific Weibel-Palade bodies. J Proteome Res 11(5):2925–2936
Kawecki C, Lenting PJ, Denis CV (2017) von Willebrand factor and inflammation. J Thromb Haemost 15(7):1285–1294
Rostami M, Mansouritorghabeh H, Parsa-Kondelaji M (2022) High levels of Von Willebrand factor markers in COVID-19: a systematic review and meta-analysis. Clin Exp Med 22(3):347–357
Aird WC (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101(10):3765–3777
Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D (2020) Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med 15(5):861–863
Xu J, Esmon NL, Esmon CT (1999) Reconstitution of the human endothelial cell protein C receptor with thrombomodulin in phosphatidylcholine vesicles enhances protein C activation. J Biol Chem 274(10):6704–6710
Walker FJ (1980) Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem 255(12):5521–5524
Mosnier LO, Zlokovic BV, Griffin JH (2007) The cytoprotective protein C pathway. Blood 109(8):3161–3172
Oto J, Fernandez-Pardo A, Miralles M, Plana E, Espana F, Navarro S, Medina P (2020) Activated protein C assays: a review. Clin Chim Acta 502:227–232
Mosnier LO, Griffin JH (2006) Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities. Front Biosci 11:2381–2399
Esmon CT (2003) The protein C pathway. Chest 124(3 Suppl):26s–32s
Healy LD, Puy C, Fernández JA, Mitrugno A, Keshari RS, Taku NA et al (2017) Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem 292(21):8616–8629
Griffin JH, Zlokovic BV, Mosnier LO (2012) Protein C anticoagulant and cytoprotective pathways. Int J Hematol 95(4):333–345
Mazzeffi MA, Chow JH, Tanaka K (2021) COVID-19 associated hypercoagulability: manifestations, mechanisms, and management. Shock 55(4):465–471
Stanne TM, Pedersen A, Gisslén M, Jern C (2021) Low admission protein C levels are a risk factor for disease worsening and mortality in hospitalized patients with COVID-19. Thromb Res 204:13–15
Stoichitoiu LE, Pinte L, Balea MI, Nedelcu V, Badea C, Baicus C (2020) Anticoagulant protein S in COVID-19: low activity, and associated with outcome. Rom J Intern Med 58(4):251–258
Hanchard J, Capó-Vélez CM, Deusch K, Lidington D, Bolz SS (2020) Stabilizing cellular barriers: raising the shields against COVID-19. Front Endocrinol (Lausanne) 11:583006
Pepper MS (2001) Extracellular proteolysis and angiogenesis. Thromb Haemost 86(1):346–355
Levin EG, Marzec U, Anderson J, Harker LA (1984) Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest 74(6):1988–1995
Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ et al (2003) Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem 278(51):51059–51067
Kolev K, Machovich R (2003) Molecular and cellular modulation of fibrinolysis. Thromb Haemost 89(4):610–621
Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B et al (2010) Molecular intercommunication between the complement and coagulation systems. J Immunol 185(9):5628–5636
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847
Ji HL, Zhao R, Matalon S, Matthay MA (2020) Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100(3):1065–1075
Kam YW, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R (2009) Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One 4(11):e7870
Antoniak S, Mackman N (2014) Multiple roles of the coagulation protease cascade during virus infection. Blood 123(17):2605–2613
Gacche RN, Gacche RA, Chen J, Li H, Li G (2021) Predictors of morbidity and mortality in COVID-19. Eur Rev Med Pharmacol Sci 25(3):1684–1707
Wu YP, Wei R, Liu ZH, Chen B, Lisman T, Ren DL et al (2006) Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemost 96(1):100–101
Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW (2020) An aberrant STAT pathway is central to COVID-19. Cell Death Differ 27(12):3209–3225
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Li B, Liu Y, Hu T, Zhang Y, Zhang C, Li T et al (2019) Neutrophil extracellular traps enhance procoagulant activity in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol 145(7):1695–1707
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V et al (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191(3):677–691
Björnsdottir H, Welin A, Michaëlsson E, Osla V, Berg S, Christenson K et al (2015) Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med 89:1024–1035
Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC (2014) Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol 34(9):1977–1984
Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT (2011) Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 9(9):1795–1803
Barranco-Medina S, Pozzi N, Vogt AD, Di Cera E (2013) Histone H4 promotes prothrombin autoactivation. J Biol Chem 288(50):35749–35757
Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I et al (1992) Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J Clin Invest 90(6):2565–2573
Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R (2001) DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 61(4):1659–1665
Nakazawa F, Kannemeier C, Shibamiya A, Song Y, Tzima E, Schubert U et al (2005) Extracellular RNA is a natural cofactor for the (auto-)activation of factor VII-activating protease (FSAP). Biochem J 385(Pt 3):831–838
Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P et al (2007) Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A 104(15):6388–6393
Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N et al (2005) Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 26(1):1–43
Strukova S (2006) Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci 11:59–80
Tunjungputri RN, Li Y, de Groot PG, Dinarello CA, Smeekens SP, Jaeger M et al (2018) The inter-relationship of platelets with interleukin-1β-mediated inflammation in humans. Thromb Haemost 118(12):2112–2125
Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC et al (2021) Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev 20(3):102763
José RJ, Williams AE, Chambers RC (2014) Proteinase-activated receptors in fibroproliferative lung disease. Thorax 69(2):190–192
Levi M, Keller TT, van Gorp E, ten Cate H (2003) Infection and inflammation and the coagulation system. Cardiovasc Res 60(1):26–39
Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10(2):102–108
Li G, Chen X, Xu A (2003) Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med 349(5):508–509
Levi M, van der Poll T (2017) Coagulation and sepsis. Thromb Res 149:38–44
Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K (2020) High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 18(7):1743–1746
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Tahereh Kalantari and Rasoul Ebrahimi had the idea for this article, Rasoul Ebrahimi performed the literature search, Rasoul Ebrahimi has written the article, and Fatemeh Nasri has critically revised the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Compliance with ethical standards
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebrahimi, R., Nasri, F. & Kalantari, T. Coagulation and Inflammation in COVID-19: Reciprocal Relationship between Inflammatory and Coagulation Markers. Ann Hematol 103, 1819–1831 (2024). https://doi.org/10.1007/s00277-024-05630-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05630-1