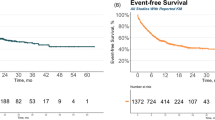
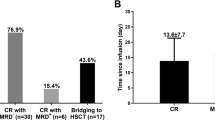
Abstract
Chimeric antigen receptor (CAR) T cell therapy improves the remission rate of refractory/relapsed B-acute lymphoblastic leukemia (R/R B-ALL) patients, but the relapse rate remains high. Recent studies suggest patients who underwent post-chimeric antigen receptor T cell therapy hematopoietic stem cell transplantation (post- HSCT) would achieve durable remission and better survival, but this remains controversial. To this end, we conducted a meta-analysis to assess the role of post-HSCT in R/R B-ALL. The Cochrane Library, Embase, and PubMed were used to identify relevant studies; the latest search update was on July 05, 2020. We used the Cochran Q test and I-squared statistics to test for heterogeneity among the studies analyzed. The fixed model and random model were used to combine results when appropriate. We performed all statistical analyses with Stata 12, and P < 0.05 was considered statistically significant. We included 18 studies with 758 patients in the meta-analysis. Our results indicated that post-HSCT was associated with lower relapse rate (RR: 0.40, 95% CI: 0.32–0.50, P = 0.000), better overall survival (HR: 0.37, 95% CI: 0.19–0.71, P = 0.003), better leukemia-free survival (HR: 0.20, 95% CI: 0.10–0.40, P = 0.000). However, post-HSCT did not influence OS in Caucasians, and CAR-T cells with CD28 co-stimulation factor bridged to HSCT did not influence OS. Post-HSCT decreased the relapse rate and improved the long-term survival of R/R B-ALL patients. R/R B-ALL patients would benefit from post-HSCT after CAR-T cell therapy.




Similar content being viewed by others
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- BM:
-
Bone marrow
- CAR-T cell:
-
Chimeric antigen receptor T cell
- CR:
-
Complete remission
- CI:
-
Confidence interval
- EFS:
-
Event-free survival
- HR:
-
Hazard ratio
- HSCT:
-
Hematopoietic stem cell transplantation
- LFS:
-
Leukemia-free survival
- MRD:
-
Minimal residual disease
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- OS:
-
Overall survival
- RR:
-
Relative risk
- post-HSCT:
-
Post-chimeric antigen receptor T cell therapy hematopoietic stem cell transplantation
- R/R B-ALL:
-
Refractory/relapsed B-acute lymphoblastic leukemia
References
Chen B, Wang YY, Shen Y, Zhang WN, He HY, Zhu YM, Chen HM, Gu CH, Fan X, Chen JM, Cao Q, Yang G, Jiang CL, Weng XQ, Zhang XX, Xiong SM, Shen ZX, Jiang H, Gu LJ, Chen Z, Mi JQ, Chen SJ (2012) Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia 26(7):1608–1616
Liu K, Chu J, Dai Y, Jiang A, Yang L, Xie Z et al (2020) (2020) Long-term follow-up of acute lymphoblastic leukemia in young children treated by the SCMC-ALL-2009 protocol. Leuk Lymphoma. 61(12):2850–2858
Vrooman LM, Blonquist TM, Harris MH, Stevenson KE, Place AE, Hunt SK, O’Brien JE, Asselin BL, Athale UH, Clavell LA, Cole PD, Kelly KM, Laverdiere C, Leclerc JM, Michon B, Schorin MA, Sulis ML, Welch JJG, Neuberg DS, Sallan SE, Silverman LB (2018) Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001. Blood Adv 2(12):1449–1458
Moricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G et al (2016) Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 127(17):2101–2112
Takahashi H, Kajiwara R, Kato M, Hasegawa D, Tomizawa D, Noguchi Y, Koike K, Toyama D, Yabe H, Kajiwara M, Fujimura J, Sotomatsu M, Ota S, Maeda M, Goto H, Kato Y, Mori T, Inukai T, Shimada H, Fukushima K, Ogawa C, Makimoto A, Fukushima T, Ohki K, Koh K, Kiyokawa N, Manabe A, Ohara A (2018) Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children's Cancer Study Group (TCCSG) Study L04-16. Int J Hematol 108(1):98–108
Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A et al (2012) Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 120(10):2032–2041
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, Xie J, Tan Y, Ni W, Tu J, Wang J, Jin A, Zhang H, Cai Z, Xiao L, Huang H (2017) Potent Anti-leukemia Activities of Chimeric Antigen Receptor-Modified T Cells against CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic Leukemia. Clin Cancer Res 23(13):3297–3306
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, Wu YN, Deng ZL, Zhang YL, Liu SH, Wu T, Lu PH, Lu DP, Chang AH, Tong CR (2017) High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 31(12):2587–2593
Wang Z, Wu Z, Liu Y, Han W (2017) New development in CAR-T cell therapy. J Hematol Oncol 10(1):53
Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, Su Y, Shi Y, Zhang M, He J, Song D, Lv F, Li W, Wu Y, Wang H, Liu H, Zhou X, He T, Lu P (2020) Efficacy and safety of anti-CD19 CAR T cell therapy in 110 patients with B cell acute lymphoblastic leukemia with high-risk features. Blood Adv 4(10):2325–2338
Jiang H, Li C, Yin P, Guo T, Liu L, Xia L, Wu Y, Zhou F, Ai L, Shi W, Lu X, Wang H, Tang L, Wei Q, Deng J, Jin R, Xiong W, Dong J, Mei H, Hu Y (2019) Anti-CD19 chimeric antigen receptor-modified T cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B cell acute lymphoblastic leukemia: an open-label pragmatic clinical trial. Am J Hematol 94(10):1113–1122
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M (2018) Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 378(5):449–459
Saadeh SS, Litzow MR (2018) Hematopoietic stem cell transplant in adults with acute lymphoblastic leukemia: the present state. Expert Rev Hematol 11(3):195–207
Giebel S, Czyz A, Ottmann O, Baron F, Brissot E, Ciceri F, Cornelissen JJ, Esteve J, Gorin NC, Savani B, Schmid C, Mohty M, Nagler A (2016) Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer 122(19):2941–2951
Bouziana S, Bouzianas D (2020) Exploring the dilemma of allogeneic hematopoietic cell transplantation after chimeric antigen receptor T cell herapy: to transplant or not? Biol Blood Marrow Transplant 26(8):e183–e191
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, Gooley TA, Cherian S, Chen X, Pender BS, Hawkins RM, Vakil A, Steinmetz RN, Schoch G, Chapuis AG, Till BG, Kiem HP, Ramos JD, Shadman M, Cassaday RD, Acharya UH, Riddell SR, Maloney DG, Turtle CJ (2019) Factors associated with durable EFS in adult B cell ALL patients achieving MRD-negative CR after CD19 CAR T cell therapy. Blood 133(15):1652–1663
Zhao H, Wei J, Wei G, Luo Y, Shi J, Cui Q, Zhao M, Liang A, Zhang Q, Yang J, Li X, Chen J, Song X, Jing H, Li Y, Hao S, Wu W, Tan Y, Yu J, Zhao Y, Lai X, Yin ETS, Wei Y, Li P, Huang J, Wang T, Blaise D, Xiao L, Chang AH, Nagler A, Mohty M, Huang H, Hu Y (2020) Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: a multi-center retrospective study. J Hematol Oncol 13(1):42
Tu S, Huang R, Guo Z, Deng L, Song C, Zhou X, Yue C, Zhang L, He Y, Yang J, Liang Z, du J, Cao P, Li Y, Chang LJ, Li Y (2019) Shortening the ex vivo culture of CD19-specific CAR T cells retains potent efficacy against acute lymphoblastic leukemia without CAR T cell-related encephalopathy syndrome or severe cytokine release syndrome. Am J Hematol 94(12):E322–E325
Hu L, Li M, Ding Y, Pu L, Liu J, Xie J, Cabanero M, Li J, Xiang R, Xiong S (2017) Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget 8(9):16027–16035
Lee DW III, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B et al (2016) Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood 128(22):218–218
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126(6):2123–2138
Zhai Z (2018) Factors influencing efficacy of CD19-CAR-T cells in children and adults with relapsed/refractory B cell lymphoblastic leukemia. Blood 132(Supplement 1):2656
Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V, Meir A, Kubi A, Levy M, Zikich D, Zeltzer LA, Brezinger K, Schachter J, Nagler A, Besser MJ, Toren A (2018) Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol 93(12):1485–1492
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, de Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA (2018) Tisagenlecleucel in children and young adults with B cell lymphoblastic leukemia. N Engl J Med 378(5):439–448
Cao J, Cheng H, Qi K, Chen W, Shi M, Zheng J, Xu K (2019) Humanized CD19-specific chimeric antigen receptor T cells for acute lymphoblastic leukemia. Blood 134:3872
Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, Kobos R, Forlenza CJ, Steinherz P, Prockop S, Boulad F, Spitzer B, Cancio MI, Boelens JJ, Kung AL, Khakoo Y, Szenes V, Park JH, Sauter CS, Heller G, Wang X, Senechal B, O’Reilly RJ, Riviere I, Sadelain M, Brentjens RJ (2019) Toxicity and response after CD19-specific CAR T cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134(26):2361–2368
Finney OC, Brakke HM, Rawlings-Rhea S, Hicks R, Doolittle D, Lopez M et al (2019) CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest 129(5):2123–2132
Zuo YX, Jia YP, Wu J, Wang JB, Lu AD, Dong LJ et al (2019) Chimeric antigen receptors T cells for treatment of 48 relapsed or refractory acute lymphoblastic leukemia children: long term follow-up outcomes. Zhonghua Xue Ye Xue Za Zhi 40(4):270–275
Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, Mangan JK, Loren AW, Perl AE, Maude SL, Grupp SA, Shah NN, Gilmore J, Lacey SF, Melenhorst JJ, Levine BL, June CH, Porter DL (2020) Optimizing chimeric antigen receptor T cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol 38(5):415–422
Wang J, Mou N, Yang Z, Li Q, Jiang Y, Meng J, Liu X, Deng Q (2020) Efficacy and safety of humanized anti-CD19-CAR-T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia. Br J Haematol 191:212–222
Liu J, Zhang X, Zhong JF, Zhang C (2017) CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B cell acute lymphoblastic leukemia. Immunotherapy 9(13):1115–1125
June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, Grupp SA, Porter DL (2014) Engineered T cells for cancer therapy. Cancer Immunol Immunother 63(9):969–975
Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, Liu Z, Zhang Y, Qu X, Zhang Y, Liu S, Ling Z, Lin Y, Zhao Y, Song Y, Tan X, Zhang Y, Li Z, Yin Z, Chen B, Yu X, Yan J, Zheng Q, Zhou X, Gao J, Chang AH, Feng X, Tong C (2019) CD22 CAR T cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33(12):2854–2866
Jia H, Wang Z, Wang Y, Liu Y, Dai H, Tong C, Guo Y, Guo B, Ti D, Han X, Yang Q, Wu Z, Han W (2019) Haploidentical CD19/CD22 bispecific CAR-T cells induced MRD-negative remission in a patient with relapsed and refractory adult B-ALL after haploidentical hematopoietic stem cell transplantation. J Hematol Oncol 12(1):57
Pehlivan KC, Duncan BB, Lee DW (2018) CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory Disease. Curr Hematol Malig Rep 13(5):396–406
Li L, Liu J, Xu M, Yu H, Lv C, Cao F, Wang Z, Fu Y, Zhang M, Meng H, Zhang X, Kang L, Zhang Z, Li J, Feng J, Lian X, Yu L, Zhou J (2020) Treatment response, survival, safety, and predictive factors to chimeric antigen receptor T cell therapy in Chinese relapsed or refractory B cell acute lymphoblast leukemia patients. Cell Death Dis 11(3):207
Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, Davis KL, Owens DK, Goldhaber-Fiebert JD (2018) Cost effectiveness of chimeric antigen receptor T cell therapy in relapsed or refractory pediatric B cell acute lymphoblastic leukemia. J Clin Oncol 36(32):3192–3202
Whittington MD, McQueen RB, Ollendorf DA, Kumar VM, Chapman RH, Tice JA, Pearson SD, Campbell JD (2018) Long-term survival and value of chimeric antigen receptor T cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr 172(12):1161–1168
Sarkar RR, Gloude NJ, Schiff D, Murphy JD (2019) Cost-effectiveness of chimeric antigen receptor T cell therapy in pediatric relapsed/refractory B cell acute lymphoblastic leukemia. J Natl Cancer Inst 111(7):719–726
Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M (2015) Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cell. Cancer Cell 28(4):415–428
Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr et al (2016) Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44(2):380–390
Salter AI, Ivey RG, Kennedy JJ, Voillet V, Rajan A, Alderman EJ et al (2018) Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal 11(544):eaat6753
Hematology Oncology Committee, C.A.-C.A., Leukemia, and C.S.o.H.C.M.A. Lymphoma Group (2016) Chinese guidelines for diagnosis and treatment of acute lymphoblastic leukemia(2016). Zhonghua Xue Ye Xue Za Zhi 37(10):837–845
Brown PA, Wieduwilt M, Logan A, DeAngelo DJ, Wang ES, Fathi A, Cassaday RD, Litzow M, Advani A, Aoun P, Bhatnagar B, Boyer MW, Bryan T, Burke PW, Coccia PF, Coutre SE, Jain N, Kirby S, Liu A, Massaro S, Mattison RJ, Oluwole O, Papadantonakis N, Park J, Rubnitz JE, Uy GL, Gregory KM, Ogba N, Shah B (2019) Guidelines insights: acute lymphoblastic leukemia, version 1.2019. J Natl Compr Cancer Netw 17(5):414–423
Inaba H, Greaves M, Mullighan CG (2013) Acute lymphoblastic leukaemia. Lancet 381(9881):1943–1955
Putter H, van Houwelingen HC (2017) Understanding landmarking and its relation with time-dependent Cox Regression. Stat Biosci 9(2):489–503
Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S (2016) Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med 35(26):4824–4836
Funding
This work was supported by the National Natural Science Foundation of China: [grant number 81670179]; Major Subject of Science and Technology of Anhui Province: [grant number 201903a07020030]; Key Research and Development Plan of Anhui Province, China (201904a07020058); and the Foundation of Anhui Medical University (2019xkj134).
Author information
Authors and Affiliations
Contributions
LHH and AC performed data collection; LHH analyzed the data and wrote the manuscript; AC, QL, WWZ, and QST, wrote the manuscript,; SDX and ZMZ wrote the manuscript and approved the final manuscript as submitted.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Hu, L., Charwudzi, A., Li, Q. et al. Anti-CD19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: a systematic review and meta-analysis. Ann Hematol 100, 1003–1012 (2021). https://doi.org/10.1007/s00277-021-04451-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04451-w