Abstract
Background
Medullary thyroid cancer (MTC) is characterized by early regional lymph node metastasis, the presence of which represents a critical obstacle to cure. At present no molecular markers have been successfully integrated into the clinical care of sporadic MTC. The present study was designed to evaluate TP53INP1 expression in MTC and to assess its ability to guide the surgeon to the optimal extent of surgery performed with curative intent.
Methods
Thirty-eight patients with sporadic MTC were evaluated. TP53INP1 immunoexpression was studied on embedded paraffin material and on cytological smears.
Results
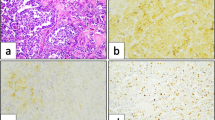
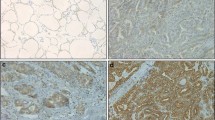
TP53INP1 was expressed in normal C cells, in C-cell hyperplasia, and in 57.9% of MTC. It was possible to identify two groups of MTC according to the proportion of TP53INP1 expressing tumor cells: group 1 from 0% to <50% and group 2 from 50% to 100% of positive cells. Patients with a decreased expression of TP53INP1 (group 1) had a lower rate of nodal metastasis (18.8% versus 63.4% in group 2; P = 0.009), with only minimal lymph node involvement per N1 patient (2.7% of positive lymph nodes versus 22.9%; P < 0.001) and better outcomes (100% of biochemical cure versus 55.5%; P < 0.001). Patients with distant metastases were only observed in group 2. Cytological samples exhibit similar results to their embedded counterparts.
Conclusions
TP53INP1 immunoexpression appears to be a clinical predictor of lymph node metastasis in MTC. The evaluation of TP53INP1 expression may guide the extent of lymph node dissection in the clinically node-negative neck. These findings require prospective validation.

Similar content being viewed by others
References
Dralle H, Scheumann GF, Proye C et al (1995) The value of lymph node dissection in hereditary medullary thyroid carcinoma: a retrospective, European, multicentre study. J Intern Med 238:357–361
Machens A, Hinze R, Thomusch O et al (2002) Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 26:22–28
Machens A, Hofmann C, Hauptmann S et al (2007) Locoregional recurrence and death from medullary thyroid carcinoma in a contemporaneous series: 5-year results. Eur J Endocrinol 157:85–93
Oskam IM, Hoebers F, Balm AJ et al (2008) Neck management in medullary thyroid carcinoma. Eur J Surg Oncol 34:71–76
Hundahl SA, Fleming ID, Fremgen AM et al (1998) A National Cancer Data Base report on 53, 856 cases of thyroid carcinoma treated in the U.S., 1985–1995 (see Comments). Cancer 83:2638–2648
Modigliani E, Cohen R, Campos JM et al (1998) Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 48:265–273
Raue F, Kotzerke J, Reinwein D et al (1993) Prognostic factors in medullary thyroid carcinoma: evaluation of 741 patients from the German Medullary Thyroid Carcinoma Register. Clin Invest 71:7–12
Machens A, Gimm O, Ukkat J et al (2000) Improved prediction of calcitonin normalization in medullary thyroid carcinoma patients by quantitative lymph node analysis. Cancer 88:1909–1915
Scollo C, Baudin E, Travagli JP et al (2003) Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 88:2070–2075
Weber T, Schilling T, Frank-Raue K et al (2001) Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery 130:1044–1049
Ellenhorn JD, Shah JP, Brennan MF (1993) Impact of therapeutic regional lymph node dissection for medullary carcinoma of the thyroid gland. Surgery 114:1078–1081 (discussion 1081–1072)
Fleming JB, Lee JE, Bouvet M et al (1999) Surgical strategy for the treatment of medullary thyroid carcinoma. Ann Surg 230:697–707
Kallinowski F, Buhr HJ, Meybier H et al (1993) Medullary carcinoma of the thyroid—therapeutic strategy derived from fifteen years of experience. Surgery 114:491–496
Kebebew E, Kikuchi S, Duh QY et al (2000) Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg 135:895–901
Machens A, Holzhausen HJ, Dralle H (2006) Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: systemic disease? Surgery 139:28–32
Moley JF, DeBenedetti MK (1999) Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 229:880–887 (discussion 887–888)
Moley JF, Fialkowski EA (2007) Evidence-based approach to the management of sporadic medullary thyroid carcinoma. World J Surg 31:946–956
Kloos RT, Eng C, Evans DB et al (2009) Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612
Iacobone M, Niccoli-Sire P, Sebag F et al (2002) Can sporadic medullary thyroid carcinoma be biochemically predicted? Prospective analysis of 66 operated patients with elevated serum calcitonin levels. World J Surg 26:886–890
Niccoli-Sire P, Murat A, Rohmer V et al (2003) When should thyroidectomy be performed in familial medullary thyroid carcinoma gene carriers with non-cysteine RET mutations? Surgery 134:1029–1036 (discussion 1036–1027)
Tamagnini P, Iacobone M, Sebag F et al (2005) Lymph node involvement in macroscopic medullary thyroid carcinoma. Br J Surg 92:449–453
Koperek O, Scheuba C, Cherenko M et al (2008) Desmoplasia in medullary thyroid carcinoma: a reliable indicator of metastatic potential. Histopathology 52:623–630
Machens A, Holzhausen HJ, Lautenschlager C et al (2003) Enhancement of lymph node metastasis and distant metastasis of thyroid carcinoma Cancer 98:712–719
Scheuba C, Kaserer K, Kaczirek K et al (2006) Desmoplastic stromal reaction in medullary thyroid cancer—an intraoperative “marker” for lymph node metastases. World J Surg 30:853–859
Gironella M, Seux M, Xie MJ et al (2007) Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA 104:16170–16175
Tomasini R, Samir AA, Carrier A et al (2003) TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity. J Biol Chem 278:37722–37729
Tomasini R, Samir AA, Pebusque MJ et al (2002) P53-dependent expression of the stress-induced protein (SIP). Eur J Cell Biol 81:294–301
Tomasini R, Seux M, Nowak J et al (2005) TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene 24:8093–8104
Herfarth KK, Wick MR, Marshall HN et al (1997) Absence of TP53 alterations in pheochromocytomas and medullary thyroid carcinomas. Genes Chromosom Cancer 20:24–29
Chevrier L, Meunier AC, Cochaud S et al (2008) Vasoactive intestinal peptide decreases MYCN expression and synergizes with retinoic acid in a human MYCN-amplified neuroblastoma cell line. Int J Oncol 33:1081–1089
Gommeaux J, Cano C, Garcia S et al (2007) Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Mol Cell Biol 27:2215–2228
Jiang PH, Motoo Y, Garcia S et al (2006) Down-expression of tumor protein p53-induced nuclear protein 1 in human gastric cancer. World J Gastroenterol 12:691–696
Jiang PH, Motoo Y, Sawabu N et al (2006) Effect of gemcitabine on the expression of apoptosis-related genes in human pancreatic cancer cells. World J Gastroenterol 12:1597–1602
Machens A, Hauptmann S, Dralle H (2008) Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg 95:586–591
Jiang PH, Motoo Y, Iovanna JL et al (2004) Tumor protein p53-induced nuclear protein 1 (TP53INP1) in spontaneous chronic pancreatitis in the WBN/Kob rat: drug effects on its expression in the pancreas. JOP 5:205–216
Ito Y, Motoo Y, Yoshida H et al (2006) Decreased expression of tumor protein p53-induced nuclear protein 1 (TP53INP1) in breast carcinoma. Anticancer Res 26:4391–4395
Ito Y, Motoo Y, Yoshida H et al (2006) High level of tumour protein p53-induced nuclear protein 1 (TP53INP1) expression in anaplastic carcinoma of the thyroid. Pathology 38:545–547
Nikiforova MN, Tseng GC, Steward D et al (2008) MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab 93:1600–1608
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Taïeb and S. Giusiano contributed equally to this work.
Rights and permissions
About this article
Cite this article
Taïeb, D., Giusiano, S., Sebag, F. et al. Tumor Protein p53-Induced Nuclear Protein (TP53INP1) Expression in Medullary Thyroid Carcinoma: A Molecular Guide to the Optimal Extent of Surgery?. World J Surg 34, 830–835 (2010). https://doi.org/10.1007/s00268-010-0395-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0395-6