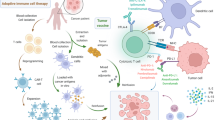
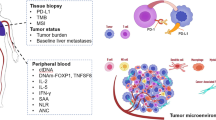
Abstract
Immunotherapy has been one of the great advances in the recent years for the treatment of advanced tumors, with nonsmall-cell lung cancer (NSCLC) being one of the cancers that has benefited most from this approach. Currently, the only validated companion diagnostic test for first-line immunotherapy in metastatic NSCLC patients is testing for programmed death ligand 1 (PD-L1) expression in tumor tissues. However, not all patients experience an effective response with the established selection criteria and immune checkpoint inhibitors (ICIs). Liquid biopsy offers a noninvasive opportunity to monitor disease in patients with cancer and identify those who would benefit the most from immunotherapy. This review focuses on the use of liquid biopsy in immunotherapy treatment of NSCLC patients. Circulating tumor cells (CTCs), cell-free DNA (cfDNA) and exosomes are promising tools for developing new biomarkers. We discuss the current application and future implementation of these parameters to improve therapeutic decision-making and identify the patients who will benefit most from immunotherapy.

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Houston KA, Henley SJ, Li J et al (2014) Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer (Amsterdam, Netherlands) 86:22–28. https://doi.org/10.1016/j.lungcan.2014.08.001
Doroshow DB, Herbst RS (2018) Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol 4:569. https://doi.org/10.1001/jamaoncol.2017.5190
Barlesi F, Mazieres J, Merlio J-P et al (2016) Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet (Lond, Engl) 387:1415–1426. https://doi.org/10.1016/S0140-6736(16)00004-0
Muinelo-Romay L, García-González J, León-Mateos L (2019) Lung cancer and liquid biopsy: realities and challenges in routine clinical practice. Arch Bronconeumol 55:289–290. https://doi.org/10.1016/j.arbres.2018.11.011
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Remon J, Passiglia F, Ahn M-J et al (2020) Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol S1556–0864:30198–30202
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Carbone DP, Reck M, Paz-Ares L et al (2017) First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med 376:2415–2426
Herbst RS, Baas P, Kim D-W et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 375:1823–1833
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 378:2078–2092
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med 379:2040–2051
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Spigel D, de Marinis F, Giaccone G et al (2019) IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol 30:v915
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301
West H, McCleod M, Hussein M et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924–937
Jotte R, Cappuzzo F, Vynnychenko I et al (2020) Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous non-small-cell lung cancer (IMpower131): results from a randomized phase III trial. J Thorac Oncol S1556–0864:30292–30296
Antonia SJ, Villegas A, Daniel D et al (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342–2350
Hellmann MD, Ciuleanu T-E, Pluzanski A et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093–2104
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020–2031
Reck M, Ciuleanu T, Cobo Dols M et al (2020) Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 38:9501–9501
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Powles T, Eder JP, Fine GD et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562
Merino DM, McShane L, Butler M et al (2019) TMB standardization by alignment to reference standards: phase II of the friends of cancer research TMB harmonization project. J Clin Oncol 37:2624–2624. https://doi.org/10.1200/JCO.2019.37.15_suppl.2624
Wood MA, Weeder BR, David JK et al (2020) Burden of tumor mutations, neoepitopes, and other variants are weak predictors of cancer immunotherapy response and overall survival. Genome Med 12:33
Yu Y, Zeng D, Ou Q et al (2019) Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open 2:e196879. https://doi.org/10.1001/jamanetworkopen.2019.6879
Rolfo C, Mack PC, Scagliotti GV et al (2018) Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol 13:1248–1268. https://doi.org/10.1016/j.jtho.2018.05.030
Corcoran RB, Chabner BA (2018) Application of cell-free DNA analysis to cancer treatment. N Engl J Med 379:1754–1765
Wan JCM, Massie C, Garcia-Corbacho J et al (2017) Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17:223–238
Blons H, Garinet S, Laurent-Puig P, Oudart JB (2019) Molecular markers and prediction of response to immunotherapy in non-small cell lung cancer, an update. J Thorac Dis 11:S25–S36
Gandara DR, Paul SM, Kowanetz M et al (2018) Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24:1441–1448
Wang Z, Duan J, Cai S et al (2019) Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 5:696–702
Chen YT, Seeruttun SR, Wu XY, Wang ZX (2019) Maximum somatic allele frequency in combination with blood-based tumor mutational burden to predict the efficacy of atezolizumab in advanced non-small cell lung cancer: a pooled analysis of the randomized POPLAR and OAK studies. Front Oncol 9:1432
Fenizia F, Pasquale R, Roma C et al (2018) Measuring tumor mutation burden in non-small cell lung cancer: tissue versus liquid biopsy. Transl Lung Cancer Res 7:668–677
Koeppel F, Blanchard S, Jovelet C et al (2017) Whole exome sequencing for determination of tumor mutation load in liquid biopsy from advanced cancer patients. PLoS ONE 12:e0188174
Stenzinger A, Allen JD, Maas J et al (2019) Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosom Cancer 58:578–588
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541:321–330
Guibert N, Jones G, Beeler JF et al (2019) Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 137:1–6
Chaudhuri AA, Chabon JJ, Lovejoy AF et al (2017) Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 7:1394–1403
Abbosh C, Birkbak NJ, Swanton C (2018) Early stage NSCLC—challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol 15:577–586
Moding EJ, Liu Y, Nabet BY et al (2020) Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer 1:176–183
Cabel L, Riva F, Servois V et al (2017) Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol 28:1996–2001
Giroux Leprieur E, Herbretau G, Dumenil C et al (2018) Circulating tumor DNA evaluated by next-generation sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. OncoImmunology 7:e1424675
Goldberg SB, Narayan A, Kole AJ et al (2018) Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res 24:1872–1880
Alama A, Coco S, Genova C et al (2019) Prognostic relevance of circulating tumor cells and circulating cell-free DNA association in metastatic non-small cell lung cancer treated with nivolumab. J Clin Med 8:1011
Alix-Panabières C, Pantel K (2016) Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 6:479–491
Hofman P, Heeke S, Alix-Panabières C, Pantel K (2019) Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol 30:1448–1459
Guibert N, Delaunay M, Lusque A et al (2018) PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 120:108–112
Boffa DJ, Graf RP, Salazar MC et al (2017) Cellular expression of PD-L1 in the peripheral blood of lung cancer patients is associated with worse survival. Cancer Epidemiol Biomark Prev 26:1139–1145
Nicolazzo C, Raimondi C, Mancini M et al (2016) Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep 6:31726
Ilié M, Szafer-Glusman E, Hofman V et al (2018) Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol 29:193–199
Dhar M, Wong J, Che J et al (2018) Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep 8:2592
Janning M, Kobus F, Babayan A et al (2019) Determination of PD-L1 expression in circulating tumor cells of NSCLC patients and correlation with response to PD-1/PD-L1 inhibitors. Cancers 11:835
Koh Y, Yagi S, Akamatsu H et al (2019) Heterogeneous expression of programmed death receptor-ligand 1 on circulating tumor cells in patients with lung cancer. Clin Lung Cancer 20:270–277
Passiglia F, Galvano A, Castiglia M et al (2019) Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther Adv Med Oncol 11:1758835919839928
Chen YL, Huang WC, Lin FM et al (2019) Novel circulating tumor cell-based blood test for the assessment of PD-L1 protein expression in treatment-naïve, newly diagnosed patients with non-small cell lung cancer. Cancer Immunol Immunother 68:1087–1094
Tamminga M, De Wit S, Hiltermann TJN et al (2019) Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immuno Therapy Cancer 7:173
Pasini L, Ulivi P (2020) Extracellular vesicles in non-small-cell lung cancer: functional role and involvement in resistance to targeted treatment and immunotherapy. Cancers 12:40
Li C, Li C, Zhi C et al (2019) Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med 17:355
Pasini L, Ulivi P (2019) Liquid biopsy for the detection of resistance mechanisms in NSCLC: comparison of different blood biomarkers. J Clin Med 8:998
Jiang T, Bai Y, Zhou F et al (2019) Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer 130:76–83
Chabon JJ, Hamilton EG, Kurtz DM et al (2020) Integrating genomic features for non-invasive early lung cancer detection. Nature 580:245–251
Hu Y, Ulrich BC, Supplee J et al (2018) False-positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res 24:4437–4443
Chan HT, Nagayama S, Chin YM et al (2020) Clinical significance of clonal hematopoiesis in the interpretation of blood liquid biopsy. Mol Oncol 14:1719–1730
Cortés-Hernández LE, Eslami-S Z, Pantel K, Alix-Panabières C (2019) Molecular and functional characterization of circulating tumor cells: from discovery to clinical application. Clin Chem 66:97–104
Rossi G, Russo A, Tagliamento M et al (2020) Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers 12:1125
Reuben A, Zhang J, Chiou SH et al (2020) Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun 11:603
Miyauchi E, Matsuda T, Kiyotani K et al (2019) Significant differences in T cell receptor repertoires in lung adenocarcinomas with and without epidermal growth factor receptor mutations. Cancer Sci 110:867–874
Funding
This study was financed by all the donors who participated in the Liquid Biopsy Crowdfunding campaign in 2017.
Author information
Authors and Affiliations
Contributions
EMBV and RDP designed the manuscript, and collected the material and data necessary to construct the article. JGG, LLM, PMM, MPC, and RLL provided supervisory support and constructive critique of the article to enable revisions. All authors helped to write the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Rafael López-López has received honoraria for participation in Advisory Boards from Roche, AstraZeneca, Merck, MSD, Bayer, BMS, Novartis, Janssen, Lilly, Pfizer and Leo; travel, accommodations and expenses from Pharmamar, Roche, BMS and Pierre Fabre; research funding from Roche and Merck; and is co-founder and shareholder in Nasasbiotech, S.L., Mtrap Inc. Luis León-Mateos reports personal fees from AstraZeneca, Boehringer-Ingelheim, Novartis, Jansen, Astellas and Sanofi; and personal fees and nonfinancial support from Bristol-Myers Squibb, Lilly, MSD and Roche, outside the submitted work. Jorge García-González reports personal fees from AstraZeneca, Boehringer-Ingelheim, Novartis, Pierre Fabre, Rovi and Sanofi; and personal fees and nonfinancial support from Bristol-Myers Squibb, Lilly, MSD and Roche, outside the submitted work. Elena Brozos-Vázquez reports personal fees from Leo Pharma, Rovi, and travel grants from Merck, Sanofi, Roche, Pierre-Frabre and Amgen, outside the submitted work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brozos-Vázquez, E.M., Díaz-Peña, R., García-González, J. et al. Immunotherapy in nonsmall-cell lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother 70, 1177–1188 (2021). https://doi.org/10.1007/s00262-020-02752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02752-z