Abstract
Purpose
The aim of this work was to demonstrate the suitability of AAZTA conjugated to PSMA inhibitor (B28110) labeled with scandium-44 as a new PET tracer for diagnostic imaging of prostate cancer.
Background
Nowadays, scandium-44 has received significant attention as a potential radionuclide with favorable characteristics for PET applications. A polyaminopolycarboxylate heptadentate ligand based on a 1,4-diazepine scaffold (AAZTA) has been thoroughly studied as chelator for Gd3+ ions for MRI applications. The excellent results of the equilibrium, kinetic, and labeling studies led to a preliminary assessment of the in vitro and in vivo behavior of [44Sc][Sc-(AAZTA)]− and two derivatives, i.e., [44Sc][Sc (CNAAZTA-BSA)] and [44Sc][Sc (CNAAZTA-cRGDfK)].
Results
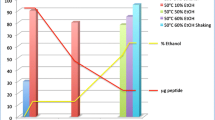
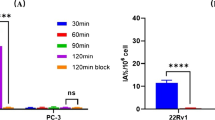
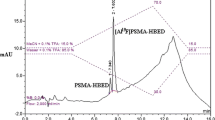
B28110 was synthesized by hybrid approach, combining solid-phase peptide synthesis (SPPS) and solution chemistry to obtain high purity (97%) product with an overall yield of 9%. Subsequently, the radioactive labeling was performed with scandium-44 produced from natural calcium target in cyclotron, in good radiochemical yields (RCY) under mild condition (pH 4, 298 K). Stability study in human plasma showed good RCP% of [44Sc]Sc-B28110 up to 24 h (94.32%). In vivo PET/MRI imaging on LNCaP tumor-bearing mice showed high tracer accumulation in the tumor regions as early as 20 min post-injection. Ex vivo biodistribution studies confirmed that the accumulation of 44Sc-PSMA-617 was two-fold lower than that of the radiolabeled B28110 probes.
Conclusions
This work demonstrated the suitability of B28110 for the complexation with scandium-44 at room temperature and the high performance of the resulting new tracer based on AAZTA chelator for the diagnosis of prostate cancer using PET.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. https://doi.org/10.3322/caac.21208.
Sam S, Chang MD. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl 10):S13–8.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Kiess A, Minn I, Chen Y, Hobbs R, Sgouros G, Mease RC, et al. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med. 2015;56:1401–7. https://doi.org/10.2967/jnumed.115.155929.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35. https://doi.org/10.1038/nrurol.2016.26.
Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL, et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52:347–57. https://doi.org/10.1021/jm800994j.
Benešová M, Bauder-Wüst U, Schäfer M, Klika KD, Mier W, Haberkorn U, et al. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–75. https://doi.org/10.1021/acs.jmedchem.5b01210.
Radchenko V, Engle JW, Medvedev DG, Maassen JM, Naranjo CM, Unc GA, et al. Proton-induced production and radiochemical isolation of. Nucl Med Biol. 2017;50:25–32. https://doi.org/10.1016/j.nucmedbio.2017.03.006.
Chaple IF, Lapi SE. Production and use of the first-row transition metal PET radionuclides. J Nucl Med. 2018;59:1655–9. https://doi.org/10.2967/jnumed.118.213264.
Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43:260–90. https://doi.org/10.1039/c3cs60304k.
Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjug Chem. 2008;19:569–73. https://doi.org/10.1021/bc700341x.
Pfister J, Summer D, Rangger C, Petrik M, von Guggenberg E, Minazzi P, et al. Influence of a novel, versatile bifunctional chelator on theranostic properties of a minigastrin analogue. EJNMMI Res. 2015;5:74. https://doi.org/10.1186/s13550-015-0154-7.
Domnanich KA, Müller C, Farkas R, Schmid RM, Ponsard B, Schibli R, et al. Sc for labeling of DOTA- and NODAGA-functionalized peptides: preclinical in vitro and in vivo investigations. EJNMMI Radiopharm Chem. 2017;1:8. https://doi.org/10.1186/s41181-016-0013-5.
Gugliotta G, Botta M, Giovenzana GB, Tei L. Fast and easy access to efficient bifunctional chelators for MRI applications. Bioorg Med Chem Lett. 2009;19:3442–4. https://doi.org/10.1016/j.bmcl.2009.05.024.
Zsolt B, Fulvio U, Alessandro M, Giovanni GB, Camilla C, Anett T, et al. Equilibrium, kinetic and structural studies of AAZTA complexes with Ga3+, In3+ and Cu2+. Eur J Inorg Chem. 2013:147–62. https://doi.org/10.1002/ejic.201201108.
Manzoni L, Belvisi L, Arosio D, Bartolomeo MP, Bianchi A, Brioschi C, et al. Synthesis of Gd and (68)Ga complexes in conjugation with a conformationally optimized RGD sequence as potential MRI and PET tumor-imaging probes. ChemMedChem. 2012;7:1084–93. https://doi.org/10.1002/cmdc.201200043.
Nagy G, Szikra D, Trencsényi G, Fekete A, Garai I, Giani AM, et al. AAZTA: an ideal chelating agent for the development of. Angew Chem Int Ed Eng. 2017;56:2118–22. https://doi.org/10.1002/anie.201611207.
Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim. 2013;42(6):217–24. https://doi.org/10.1038/laban.254.
Bunka M, Müller C, Vermeulen C, Haller S, Türler A, Schibli R, et al. Imaging quality of (44)Sc in comparison with five other PET radionuclides using Derenzo phantoms and preclinical PET. Appl Radiat Isot. 2016;110:129–33. https://doi.org/10.1016/j.apradiso.2016.01.006.
Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, et al. Matching the decay half-life with the biological half-life: ImmunoPET imaging with (44)Sc-labeled cetuximab Fab fragment. Bioconjug Chem. 2014;25:2197–204. https://doi.org/10.1021/bc500415x.
Conti M, Eriksson L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys. 2016;3:8. https://doi.org/10.1186/s40658-016-0144-5.
Gorges TM, Riethdorf S, von Ahsen O, Nastał YP, Röck K, Boede M, et al. Heterogeneous PSMA expression on circulating tumor cells: a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget. 2016;7:34930–41. https://doi.org/10.18632/oncotarget.9004.
Müller C, Domnanich KA, Umbricht CA, van der Meulen NP. Scandium and terbium radionuclides for radiotheranostics: current state of development towards clinical application. Br J Radiol. 2018;91:20180074. https://doi.org/10.1259/bjr.20180074.
Ray Banerjee S, Chen Z, Pullambhatla M, Lisok A, Chen J, Mease RC, et al. Preclinical comparative study of (68)Ga-labeled DOTA, NOTA, and HBED-CC chelated radiotracers for targeting PSMA. Bioconjug Chem. 2016;27:1447–55. https://doi.org/10.1021/acs.bioconjchem.5b00679.
Nagy G, Dénes N, Kis A, Szabó JP, Berényi E, Garai I, et al. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific. Eur J Pharm Sci. 2017;106:336–44. https://doi.org/10.1016/j.ejps.2017.06.026.
Acknowledgments
We thank Lorenzo Tei for supporting synthesis activity.
Funding
The research was totally supported by Bracco Imaging SpA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and informed consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the Hungarian Laws and regulations of the European Union (Permission numbers: animal house: XXVIII-KÁT/2015, ethical license for the study: 19/2017/DE MÁB). Approval for this study was granted by the Scientific Ethics Council of Animal Experiments.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
ESM 1
(DOCX 509 kb).
Rights and permissions
About this article
Cite this article
Ghiani, S., Hawala, I., Szikra, D. et al. Synthesis, radiolabeling, and pre-clinical evaluation of [44Sc]Sc-AAZTA conjugate PSMA inhibitor, a new tracer for high-efficiency imaging of prostate cancer. Eur J Nucl Med Mol Imaging 48, 2351–2362 (2021). https://doi.org/10.1007/s00259-020-05130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05130-0