Abstract
Purpose
To assess the brain metabolic correlates of the different regional extent of ALS, evaluated with the King’s staging system, using brain 18F-2-fluoro-2-deoxy-d-glucose-PET (18F-FDG-PET).
Methods
Three hundred ninety ALS cases with King’s stages 1, 2, and 3 (n = 390), i.e., involvement of 1, 2, and 3 body regions respectively, underwent brain 18F-FDG-PET at diagnosis. King’s stage at PET was derived from ALSFRS-R and was regressed out against whole-brain metabolism in the whole sample. The full factorial design confirmed the hypothesis that differences among groups (King’s 1, King’s 2, King’s 3, and 40 healthy controls (HC)) existed overall. Comparisons among stages and between each group and HC were performed. We included age at PET and sex as covariates.
Results
Brain metabolism was inversely correlated with stage in medial frontal gyrus bilaterally, and right precentral and postcentral gyri. The full factorial design resulted in a significant main effect of groups. There was no significant difference between stages 1 and 2. Comparing stage 3 to stage 1+2, a significant relative hypometabolism was highlighted in the former in the left precentral and medial frontal gyri, and in the right medial frontal, postcentral, precentral, and middle frontal gyri. The comparisons between each group and HC showed the extension of frontal metabolic changes from stage 1 to stage 3, with the larger metabolic gap between stages 2 and 3.
Conclusions
Our findings support the hypothesis that in ALS, the propagation of neurodegeneration follows a corticofugal, regional ordered pattern, extending from the motor cortex to posterior and anterior regions.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting upper and lower motor neurons, leading to muscle weakness and wasting that progressively spreads across body regions. In about 50% of cases, also prefrontal regions are involved, causing various degrees of cognitive impairment of the frontotemporal type [1]. Death usually occurs within 2–5 years due to respiratory failure [2]. It has been proposed that ALS pathology disseminates in a regional ordered sequence, following a cortico-efferent spreading model [3].
In recent years, King’s staging system has been proposed for ALS, mainly based on the extension of the disease across body regions [4]. It defines the following stages: 1, symptom onset (involvement of the first region); 2A, diagnosis; 2B, involvement of a second region; 3, involvement of a third region; 4A, need for gastrostomy; 4B, need for respiratory support (non-invasive ventilation). Central nervous system region involvement was defined by the presence of weakness, wasting, spasticity, dysphagia, or dysarthria. Regions were defined in the same way as for the El Escorial criteria [5]. Afterwards, an algorithm based on ALSFRS-R has been proposed to estimate King’s stages [6].
Published data about the neuroimaging correlates of King’s stages are limited to few magnetic resonance imaging (MRI) studies based on small samples [7,8,9]. The aim of this study was to assess the brain metabolic correlates of a different regional extent of ALS, evaluated according to King’s staging system, using the brain 18F-2-fluoro-2-deoxy-d-glucose-PET (18F-FDG-PET) since it is a measure of neuronal injury and degeneration in vivo [10].
Materials and methods
Study participants
Patients diagnosed with definite, probable, and probable laboratory-supported ALS according to El Escorial revised diagnostic criteria [5], who underwent brain 18F-FDG-PET at diagnosis between 2008 and 2015 at the ALS Centre of Turin (“Rita Levi Montalcini” Department of Neuroscience, University of Turin, Turin, Italy) were considered eligible for the study (n = 406). The present study includes patients whose brain 18F-FDG-PET scan was included in the analyses performed in previous publications.
Forty healthy controls (HC) were included. Subjects who were referred to the PET center for suspected lung cancer but in whom no oncologic disease was detected with 18F-FDG-PET/CT and who had a normal neurological assessment were considered eligible as controls. Otherwise, major systemic illnesses, major vision disturbances, psychiatric illnesses, and diseases affecting brain functioning and metabolism represented exclusion criteria.
Genetic analysis
All patients underwent genetic analysis for C9ORF72, SOD1, TARDBP, and FUS genes. All the coding exons and 50 bp of the flanking intron-exon boundaries of SOD1, of exon 6 of TARDBP, and of exons 14 and 15 of FUS have been PCR amplified, sequenced using the BigDye Terminator v3.1 sequencing kit (Applied Biosystems Inc.), and run on an ABIPrism 3500 genetic analyzer. These exons were selected as the vast majority of known pathogenic variants are known to lie within these mutational hotspots. A repeat-primed PCR assay was used to screen for the presence of the GGGGCC hexanucleotide expansion in the first intron of C9ORF72.
18F-FDG-PET acquisition
18F-FDG-PET was performed according to published guidelines [11]. Patients fasted at least 6 h before the exam. Blood glucose was < 7.2 mmol/l in all cases before the procedure. After a 20-min rest, about 185 MBq of 18F-FDG was injected. The acquisition started 60 min after the injection. In the patient group, a whole-body scan was performed setting head first. In the control group, a separate brain scan was performed after the whole-body one with a time difference of 15 min. The 18F-FDG-PET acquisition procedure was performed in the same environmental conditions in patients and controls, according to published guidelines [11]. PET/CT scans were performed on a Discovery ST-E System (General Electric). Brain CT (slice thickness of 3.75 mm, 140 kV, 60–80 mAs) and PET scan were sequentially acquired, the former being used for attenuation correction of PET data. The PET images were reconstructed with 4 iterations and 28 subsets with an initial voxel size of 2.34 × 2.34 × 2.00 mm and data were collected in 128 × 128 matrices.
Assessment of King’s stage
King’s staging is based on the spreading of motor symptoms in three different body regions (bulbar, upper limbs, and lower limbs) and on the use of non-invasive ventilation (NIV) and enteral nutrition. We used the algorithm proposed by Balendra et al. [6] to calculate King’s stage from ALSFRS-R. The bulbar region was considered involved if a patient lost any points on any of the three items regarding speech, salivation, or swallowing (items 1, 2, and 3). The upper limb region was considered involved if a patient lost any points on either of the two items regarding handwriting and ability to cut food and handle utensils (items 4 and 5A). The lower limb region was considered involved if a patient lost any points on the item regarding walking (item 8). The presence of gastrostomy was confirmed by the assessment of item 5B (evaluation of the ability to manipulate fastenings if a patient has a gastrostomy) rather than item 5A (answered by patients without gastrostomy). If a subject scored 0 points on question 10 (indicating that the patient has significant difficulty with dyspnea and is considering using mechanical respiratory support) or less than 4 points on question 12 (dropping any points on this question indicates that Bi-level airway pressure ventilation is being used), this indicated that the patient was using NIV. We classified patients according to the following five stages of King’s staging system: 1, one region involved; 2, two regions involved; 3, three regions involved; 4A, patient needs gastrostomy; 4B, patient needs NIV.
Since our aim was to evaluate the metabolic changes related to the different regional extent of ALS, we focused on patients classified as King’s stages 1, 2, and 3 (n = 390). Indeed, stages 1, 2, and 3 correspond to involvement of 1, 2, and 3 body regions respectively, whereas stages 4A and 4B correspond to the reaching of milestones related to functional impairment (i.e., gastrostomy and NIV).
Statistical analysis
The demographic and clinical characteristics of patient groups (King’s stages 1, 2, and 3) and HC were compared as follows. The χ2 test was employed for categorical variables. The analysis of variance (ANOVA) or the Kruskal-Wallis test was used for quantitative, continuous variables. The homogeneity assumption needed for ANOVA was evaluated through Levene’s test. In the case of significant Levene’s test, the Kruskal-Wallis test was employed instead of ANOVA.
SPM12 implemented in Matlab R2018b (MathWorks, Natick, MA, USA) was used for image spatial normalization to a customized brain 18F-FDG-PET template [12]. Intensity normalization was performed using the 0.8 default SPM value of gray matter threshold and images were subsequently smoothed with a 10-mm filter and submitted to statistical analysis.
In our sample (n = 390), King’s stage (1, 2, 3) was regressed out against whole-brain metabolism. The SPM12 multiple regression routine was implemented with age at PET and sex as covariates and the height threshold was set at p < 0.001 (p < 0.05 FWE corrected at cluster level). We used the full factorial design as implemented in SPM12 to test the hypothesis that differences among groups (King’s 1, King’s 2, King’s 3, HC) exist overall (i.e., main effect of groups). In case the hypothesis was confirmed, comparisons between groups defined according to King’s stage were performed through the two-sample t test model of SPM12. For both analyses, age at PET and sex were used as covariates and the height threshold was set at p < 0.001 (p < 0.05 FWE corrected at cluster level). Although the scope of the study was the assessment of metabolic differences across King’s stages, for a more exhaustive characterization of patient metabolic state, each group defined according to King’s stage was compared with HC, through the two-sample t test model of SPM12, with age at PET and sex as covariates, setting the height threshold at p < 0.0001 (p < 0.05 FWE corrected at cluster level). In all the analyses, only clusters containing > 125 contiguous voxels were considered significant. Brodmann areas (BAs) were identified at a 0–2-mm range from the Talairach coordinates of the SPM output isocenters corrected by Talairach Client (http://www.talairach.org/index.html).
Results
Demographic and clinical data
Based on the ALSFRS-R at the time of PET, 165 patients (42.3%) were in King’s stage 1, 133 (34.1%) in stage 2, and 92 (23.6%) in stage 3. The demographic and clinical characteristics of patients belonging to the three groups and of the whole sample are reported in Table 1. We found a significant difference among the three groups for the site of onset (p < 0.001), ALSFRS-R total score (p < 0.001), and cognitive status as defined by diagnostic criteria published by Strong et al. [13] (p = 0.019). However, a bivariate correlation analysis did not show any correlation between the site of onset and King’s stage (p = 0.36). Regarding the difference in ALSFRS-R total score, this could be expected since King’s stage has been calculated based on the ALSFRS-R score according to the algorithm published by Balendra et al. [6]. As for the differences in cognitive status, these confirm a previous study showing that cognitive impairment in ALS tends to be more severe as King’s clinical stage increases [14].
In the HC group, the median age was 66.5 years (interquartile range 55.0–72.0), and the male/female ratio was 2.64 (29/11). In the comparisons between each stage group and HC, we found no significant difference for age and sex distribution, with the exception of sex distribution between King’s stage 3 and HC (p = 0.002). Nevertheless, both age and sex were included as covariates in the analyses.
18F-FDG-PET data
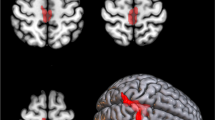
Using multiple regression analysis, we found an inverse correlation between brain metabolism and King’s stage (with stage 3 on the relatively hypometabolic side and stage 1 on the relatively hypermetabolic side). We identified clusters including the bilateral medial frontal gyrus (Brodmann area, BA, 6), right precentral gyrus (BA 4), and right postcentral gyrus (BA 2) (Fig. 1, Table 2). We did not identify any cluster with a positive correlation.
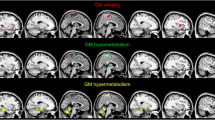
The full factorial design resulted in a significant main effect of groups (Supplemental Fig. 1). We hence computed the post hoc comparisons between the four groups. Comparing King’s stage 1 and King’s stage 2 groups, we did not find any significant difference. Therefore, we merged such groups into King’s 1+2 group. The comparison between King’s 1+2 group and the King’s 3 group revealed a significant relative hypometabolism in King’s 3 group in clusters including left precentral and medial frontal cortex (BAs 4 and 6), and right medial frontal, postcentral, precentral, and middle frontal cortex (BAs 4, 6, 3, 9, and 8) (Fig. 2, Table 3). King’s 3 group did not show any cluster of relative hypermetabolism as compared with King’s 1+2 group.
In the comparison with HC, we identified two clusters of relative hypometabolism in patient groups. The former included occipito-temporo-parietal regions and did not show a clear pattern of extension along with the increase of the clinical stage. The latter included frontal regions and showed an extension from left to right hemisphere from King’s stage 1 to stage 2 and a marked bilateral extension in stage 3 (Supplemental Figures 2, 3, and 4). No clusters of relative hypermetabolism in patient groups as compared with HC were found.
Discussion
In this study, we explored the 18F-FDG-PET correlates of ALS clinical stages according to King’s staging system. We focused on King’s stages 1, 2, and 3, corresponding to the spreading of motor impairment to 1, 2, and 3 body regions respectively. A full factorial analysis showed that group differences exist at a global level and overlap the ones found between groups and groups and controls.
In the multiple regression analysis, brain metabolism negatively correlated with King’s stage. We found a decreasing gradient of metabolism going from King’s stage 1 to King’s stage 3 in clusters including right precentral and postcentral gyrus (BAs 4 and 2) and bilateral medial frontal gyrus (BA 6). When performing group comparisons, no significant difference was detected between King’s stage 1 group and King’s stage 2 group. King’s stage 3 group showed relatively hypometabolic clusters when compared with King’s stage 1+2 group, including left precentral and medial frontal gyrus (BAs 4 and 6) and right precentral, middle frontal, postcentral, and medial frontal gyrus (BAs 4, 6, 3, 8, and 9). The comparisons between each stage group and HC resulted in agreement with such findings, showing the extension of frontal metabolic changes from stage 1 to stage 3, with the larger metabolic gap between stages 2 and 3. Posterior clusters, including occipital lobes in all comparisons, of relative hypometabolism in ALS patients as compared with HC have already been reported [15].
The corticospinal tracts (CST) originate from neurons mainly situated in BA 4, corresponding to the primary motor cortex. Nevertheless, CST fibers rise also from neurons situated in other cortical regions, including the premotor and supplementary motor cortex (BA 6), and primary somatosensory cortex (BAs 1, 2, and 3). Therefore, we identified a decreasing gradient of metabolism going from patients with King’s stage 1 to subjects with King’s stage 3 in cortical regions from which the corticospinal tracts originate. Furthermore, group comparisons suggested that the main metabolic gap is situated between stages 2 and 3. We detected no difference between subjects with King’s stages 1 and 2. A possible explanation is that patients showing the involvement of three body regions at diagnosis subtend a more rapid neurodegenerative process, involving the prefrontal cortex to a greater extent, as compared with patients with stages 1 and 2. Otherwise, the metabolic difference between stages 1 and 2 is probably under the threshold, being the neurodegenerative process slower and less extensive.
Clusters of relative hypometabolism in subjects with King’s stage 3 as compared with patients with stages 1 and 2 included BAs 4, 6, and 3 and extended towards anterior regions (BAs 8 and 9). In a previous study, we identified a decreasing gradient of metabolism going from ALS with normal cognition to ALS with FTD, through cases with intermediate cognitive deficits (ALS-Ci) in frontal clusters including BAs 8 and 9 [16]. Synapse degeneration in BA 9 has been reported as a strong predictor of cognitive impairment in ALS [17]. Our findings are in agreement with neuropathological data suggesting that phosphorylated TDP-43 (p-TDP-43) tends to spread from the motor cortex to prefrontal and postcentral regions via axonal pathways [3]. According to such staging of p-TDP-43 pathology in ALS, BAs 4 and 6 are involved in stage 1, while the extension to the prefrontal and postcentral cortex is reported in stage 3. The data of the present study seem to strengthen the findings of our previous population-based cross-sectional study on cognitive impairment across ALS clinical stages [14], suggesting that cognitive deficits tend to be more severe as the clinical stage increases. In the proposed staging of p-TDP-43 pathology [3], the neurodegenerative process was thought to spread along axonal pathways. Our results are in agreement with such hypothesis that is further strengthened by several MRI studies, aimed at tracking the spreading of the neurodegenerative process in vivo through diffusion tensor imaging (DTI) [18,19,20], and simulation models based on brain network analyses [21, 22].
A 18F-FDG-PET study [23] on 146 patients evaluated the possible correspondence between the key brain regions used in the assessment of Brettschneider’s neuropathological staging system [3] and metabolic patterns allowing in vivo staging of ALS. This study found that the post-mortem neuropathological stages corresponded to distinct metabolic patterns.
The cross-sectional design of our study may have limited our findings. Nevertheless, patients were tested early after diagnosis and the cognitive impairment at that time point likely reflects the speed of lesion spreading to non-motor cortical areas of the brain. Besides, we can assume that King’s stages come in succession along the disease course. Indeed, in a recent study using databases from two multicenter clinical trials, 725 patients were retrospectively staged through the trial course. The authors reported that no reversions to earlier disease stages occurred and that most people progressed to the consecutive stage [24]. In ALS, longitudinal studies on neuroimaging are challenging since disability worsens over time: patients may be unable to undergo follow-up scans due to severe motor impairment and/or the development of respiratory failure. A recent review [25] evaluated published studies employing neuroimaging to track the course of ALS but did not report any 18F-FDG-PET study.
Another possible limitation should be considered in the interpretation of the asymmetry of the metabolic clusters identified in the analyses, due to the lacking of full information about handedness. Indeed, such data were available only for a part of the patient sample and were unavailable for controls. A further possible limitation is the absence of partial volume effect correction for cortical atrophy. Unfortunately, MRI scans were not available for all subjects. However, studies employing voxel-based atrophy correction of resting glucose metabolism showed that metabolic measurements were relatively independent of brain atrophy [26].
On the other hand, our study increases the knowledge about the neuroimaging correlates of King’s stages, which is still limited to few MRI studies with small samples. In this field, a recent study using magnetic resonance (MR) high-angular resolution diffusion imaging (HARDI), suggested an early pattern of microstructural degeneration in ALS, mainly involving the CST and the corpus callosum [7]. Nevertheless, in such study, patients with early disease stages were not compared with cases with more advanced stages. A further study investigated connectivity alterations associated with different stages of ALS [8], employing magnetoencephalography and MRI. The authors suggested that modifications, in terms of increased connectedness of functional brain networks, are related to disease progression. These results further support the hypothesis of the multi-system involvement of brain networks in ALS. Finally, a longitudinal study [9] reported that King’s ALS disease stages estimated from the ALSFRS-R correlated with CST diffusion measures in C9orf72 carriers with heterogeneous clinical presentations (asymptomatic subjects, and ALS, ALS-FTD, and bvFTD patients). Based on the limited number of mutation carriers in our series, we think that the possible impact of genetic mutations on brain metabolism should be investigated in further studies including a larger amount of carriers.
Another strength of this study is the contribution to the debate about the use of brain 18F-FDG-PET in ALS. Recently, a panel of experts, on behalf of the European Association of Nuclear Medicine (EANM) and the European Academy of Neurology (EAN), did not recommend the clinical use of brain 18F-FDG-PET even to study ALS-related brain dysfunction [27, 28]. We feel that the present data support the use of 18F-FDG-PET to study ALS-related brain changes across motor and cognitive regions in a research setting. Otherwise, in agreement with the EANM-EAN recommendations, its use in the clinical setting needs further studies, including the comparison with ALS mimic disorders, to achieve a reliable accuracy at the single-patient level.
To our knowledge, this is the first study evaluating the brain metabolic correlates of regional extent expressed as disease staging in ALS and includes the largest ALS series with 18F-FDG-PET assessment ever published. We found that, with the increase of King’s stage, there was a decrease in metabolism in motor areas, with progressive involvement of extramotor regions. Since 18F-FDG-PET is a marker of neurodegeneration in vivo [10] and is considered valuable in cross-sectionally evaluating the spread of lesions in ALS [29], our data are in keeping with the ALS neuropathological staging model supporting that neurodegeneration extends from the motor cortex to posterior and anterior regions, possibly via axonal pathways [3], and are in agreement with our population-based, cross-sectional data showing that cognitive impairment tends to be more severe as the clinical stage increases [14]. Longitudinal studies are indispensable for the correlation of brain metabolic alterations with the progressive clinical involvement of different body regions and the possible cognitive deterioration over time.
Data availability
Data will be available upon request by interested researchers.
References
Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168–73.
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, et al. Amyotrophic lateral sclerosis. Lancet Lond Engl. 2017;390:2084–98.
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38.
Roche JC, Rojas-Garcia R, Scott KM, Scotton W, Ellis CE, Burman R, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain J Neurol. 2012;135:847–52.
Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, Amyotroph Lateral Scler Mot Neuron Disord Off Publ World Fed Neurol Res Group Mot Neuron Dis. 2000;1:293–9.
Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Front Degener. 2014;15:279–84.
Trojsi F, Caiazzo G, Di Nardo F, Fratello M, Santangelo G, Siciliano M, et al. High angular resolution diffusion imaging abnormalities in the early stages of amyotrophic lateral sclerosis. J Neurol Sci. 2017;380:215–22.
Sorrentino P, Rucco R, Jacini F, Trojsi F, Lardone A, Baselice F, et al. Brain functional networks become more connected as amyotrophic lateral sclerosis progresses: a source level magnetoencephalographic study. NeuroImage Clin. 2018;20:564–71.
Floeter MK, Danielian LE, Braun LE, Wu T. Longitudinal diffusion imaging across the C9orf72 clinical spectrum. J Neurol Neurosurg Psychiatry. 2018;89:53–60.
Jack CR, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Lowe V, et al. Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol. 2012;69:856–67.
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F] FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–93.
Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Front Degener. 2017;18:153–74.
Chiò A, Moglia C, Canosa A, Manera U, Vasta R, Brunetti M, et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology. 2019;93:e984–94.
Pagani M, Chiò A, Valentini MC, Öberg J, Nobili F, Calvo A, et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. 2014;83:1067–74.
Canosa A, Pagani M, Cistaro A, Montuschi A, Iazzolino B, Fania P, et al. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology. 2016;86:44–9.
Henstridge CM, Sideris DI, Carroll E, Rotariu S, Salomonsson S, Tzioras M, et al. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol (Berl). 2018;135:213–26.
Kassubek J, Müller H-P, Del Tredici K, Brettschneider J, Pinkhardt EH, Lulé D, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain J Neurol. 2014;137:1733–40.
Müller H-P, Turner MR, Grosskreutz J, Abrahams S, Bede P, Govind V, et al. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:570–9.
Kassubek J, Müller H-P, Del Tredici K, Lulé D, Gorges M, Braak H, et al. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J Neurol Neurosurg Psychiatry. 2018;89:374–81.
Schmidt R, de Reus MA, Scholtens LH, van den Berg LH, van den Heuvel MP. Simulating disease propagation across white matter connectome reveals anatomical substrate for neuropathology staging in amyotrophic lateral sclerosis. NeuroImage. 2016;124:762–9.
Meier JM, van der Burgh HK, Nitert AD, Bede P, de Lange SC, Hardiman O, et al. Connectome-based propagation model in amyotrophic lateral sclerosis. Ann Neurol. 2020;87:725–38.
van Weehaeghe D, Ceccarini J, Willekens SM, de Vocht J, van Damme P, van Laere K. Is there a glucose metabolic signature of spreading TDP-43 pathology in amyotrophic lateral sclerosis? Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Of. 2017;
Balendra R, Jones A, Jivraj N, Steen IN, Young CA, Shaw PJ, et al. Use of clinical staging in amyotrophic lateral sclerosis for phase 3 clinical trials. J Neurol Neurosurg Psychiatry. 2015;86:45–9.
Chipika RH, Finegan E, Li Hi Shing S, Hardiman O, Bede P. Tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Front Neurol. 2019;10:229.
Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50:1585–93.
Nobili F, Arbizu J, Bouwman F, Drzezga A, Agosta F, Nestor P, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25:1201–17.
Agosta F, Altomare D, Festari C, Orini S, Gandolfo F, Boccardi M, et al. Clinical utility of FDG-PET in amyotrophic lateral sclerosis and Huntington’s disease. Eur J Nucl Med Mol Imaging. 2018;45:1546–56.
Kassubek J, Pagani M. Imaging in amyotrophic lateral sclerosis: MRI and PET. Curr Opin Neurol. 2019;32:740–6.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This study was in part supported by a grant from the Thierry Latran Foundation (INSPIRED project), by the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, grant RF-2016-02362405), the European Commission’s Health Seventh Framework Programme (FP7/2007-2013 under grant agreement 259867), the Italian Ministry of Education, University and Research (Progetti di Ricerca di Rilevante Interesse Nazionale, PRIN, grant 2017SNW5MB), and the Joint Programme - Neurodegenerative Disease Research (Strength and Brain-Mend projects), granted by Italian Ministry of Education, University and Research. This study was performed under the Department of Excellence grant of the Italian Ministry of Education, University and Research to the “Rita Levi Montalcini” Department of Neuroscience, University of Turin, Italy. Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Study concept and design: Antonio Canosa, Koen Van Laere, Philip Van Damme, Marco Pagani, and Adriano Chiò. Acquisition of data: Antonio Canosa, Andrea Calvo, Cristina Moglia, Umberto Manera, Rosario Vasta, Francesca Di Pede, Sara Cabras, Vincenzo Arena, Maurizio Grassano. Analysis and interpretation of data: Antonio Canosa, Andrea Calvo, Cristina Moglia, Francesca Di Pede, Davide Nardo, Fabrizio D’Ovidio, Koen Van Laere, Philip Van Damme, Marco Pagani, Adriano Chiò. Drafting of the manuscript: Antonio Canosa, Francesca Di Pede, Marco Pagani, and Adriano Chiò. Critical revision of the manuscript for important intellectual content: Antonio Canosa, Andrea Calvo, Cristina Moglia, Umberto Manera, Rosario Vasta, Francesca Di Pede, Sara Cabras, Davide Nardo, Vincenzo Arena, Maurizio Grassano, Fabrizio D’Ovidio, Koen Van Laere, Philip Van Damme, Marco Pagani, and Adriano Chiò. Obtained funding: Koen Van Laere, Philip Van Damme, Marco Pagani, and Adriano Chiò. Administrative, technical, and material support: Andrea Calvo, Cristina Moglia, Umberto Manera, Rosario Vasta, Francesca Di Pede, Sara Cabras, Davide Nardo, Vincenzo Arena, Maurizio Grassano, Fabrizio D’Ovidio. Study supervision: Antonio Canosa, Andrea Calvo, Marco Pagani, and Adriano Chiò. Antonio Canosa had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the submitted version of the article. The information reported in the manuscript has never been reported elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
Antonio Canosa, Cristina Moglia, Umberto Manera, Rosario Vasta, Francesca Di Pede, Sara Cabras, Davide Nardo, Vincenzo Arena, Maurizio Grassano, Fabrizio D’Ovidio, and Marco Pagani report no disclosures. Andrea Calvo has received a research grant from Cytokinetics. Koen Van Laere and Philip Van Damme hold a senior clinical investigatorship of FWO-Vlaanderen. Philip Van Damme is supported by E. von Behring Chair for Neuromuscular and Neurodegenerative Disorders, the ALS Liga België, and the KU Leuven funds “Een Hart voor ALS,” “Laeversfonds voor ALS Onderzoek,” and the “Valéry Perrier Race against ALS Fund.” Adriano Chiò serves on scientific advisory boards for Mitsubishi Tanabe, Roche, Biogen, Cytokinetics, and AveXis, and has received a research grant from Italfarmaco.
Protocol approvals and informed consent
The study was approved by the ethical committee “Comitato Etico Interaziendale Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino.” The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patients signed a written informed consent. They did not receive any remuneration for participation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology.
Electronic supplementary material
Supplemental Figure 1
Glass brain rendering of the full factorial analysis including the following groups: King’s stage 1, King’s stage, 2, King’s stage 3, and healthy controls. The clusters showing a significant main effect of groups are projected on brain surface. (PNG 801 kb)
Supplemental Figure 2
Glass brain rendering of the comparison: King’s stage 1 versus healthy controls. The clusters showing a statistically significant relative hypometabolism in the King’s stage 1 group as compared to healthy controls are projected on brain surface. (PNG 768 kb)
Supplemental Figure 3
Glass brain rendering of the comparison: King’s stage 2 versus healthy controls. The clusters showing a statistically significant relative hypometabolism in the King’s stage 2 group as compared to healthy controls are projected on brain surface. (PNG 731 kb)
Supplemental Figure 4
Glass brain rendering of the comparison: King’s stage 3 versus healthy controls. The clusters showing a statistically significant relative hypometabolism in the King’s stage 3 group as compared to healthy controls are projected on brain surface. (PNG 1265 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Canosa, A., Calvo, A., Moglia, C. et al. Brain metabolic changes across King’s stages in amyotrophic lateral sclerosis: a 18F-2-fluoro-2-deoxy-d-glucose-positron emission tomography study. Eur J Nucl Med Mol Imaging 48, 1124–1133 (2021). https://doi.org/10.1007/s00259-020-05053-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05053-w