Abstract
Purpose
To evaluate parameters derived from 68Ga-PSMA-11 PET/CT images for discriminating pathological characteristics in primary clear-cell renal cell carcinoma (ccRCC).
Methods
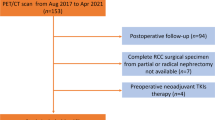
The study retrospectively examined data for 36 ccRCC patients with preoperative 68Ga-PSMA-11 PET/CT scan and surgical specimens. Radiological parameters including maximal tumor diameter, mean CT value, and maximal standard uptake value (SUVmax) were derived from PET/CT images. Pathological characteristics included WHO/ISUP grade and adverse pathology (tumor necrosis or sarcomatoid or rhabdoid feature). Values of radiological parameters were compared within subgroups of pathological characteristics. Receiver operating characteristic (ROC) curve analysis was used for the effectiveness of radiological parameters in differentiating pathological characteristics, estimating area under the ROC curve (AUC) and 95% confidence intervals (CIs).
Results
The WHO/ISUP grade distribution for 36 tumors was grade 1, 9 (25.0%); grade 2, 12 (33.3%); grade 3, 9 (25.0%); and grade 4, 6 (16.7%). Adverse pathology was positive for 15 (41.7%). Radiological tumor diameter and SUVmax significantly differed by WHO/ISUP grade, pT stage, and adverse pathology (all P < 0.05), with no difference by CT value. Tumor diameter demonstrated sensitivity 86% and specificity 88% for pT stage, with cutoff 6.70 and AUC 0.91 (95% CI, 0.79–1.00, P < 0.001). SUVmax could effectively differentiate WHO/ISUP grade (3–4 vs. 1–2) and adverse pathology (positive vs. negative), with AUC 0.89 (95% CI, 0.81–0.98, P < 0.001), cutoff 16.4, sensitivity 100%, and specificity 71% and AUC 0.92 (95% CI, 0.85–0.99, P < 0.001), cutoff 18.5, sensitivity 94%, and specificity 87%, respectively.
Conclusion
68Ga-PSMA-11 PET/CT could effectively identify aggressive pathological features of ccRCC.




Similar content being viewed by others
Abbreviations
- AUC:
-
area under the curve
- ccRCC:
-
clear-cell renal cell carcinoma
- CI:
-
confidence interval
- IHC:
-
immunohistochemistry
- PET/CT:
-
positron emission tomography/computed tomography
- PSMA:
-
prostate-specific membrane antigen
- RCC:
-
renal cell carcinoma
- ROC:
-
receiver operating characteristic curve
- ROIs:
-
regions of interest
- SUVmax :
-
maximal standard uptake value
- WHO/ISUP grade:
-
World Health Organization/International Society of Urological Pathology grade
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. https://doi.org/10.3322/caac.21442.
Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. https://doi.org/10.1016/j.eururo.2018.08.036.
Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188:391–7. https://doi.org/10.1016/j.juro.2012.04.006.
Delahunt B, Eble JN, Egevad L. Grading of renal cell carcinoma. Histopathology. 2019;74:4–17. https://doi.org/10.1111/his.13735.
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. https://doi.org/10.1016/j.eururo.2016.02.029.
Dagher J, Delahunt B. Clear cell renal cell carcinoma: validation of World Health Organization/International Society of Urological Pathology grading. Histopathology. 2017;71:918–25. https://doi.org/10.1111/his.13311.
Delahunt B, McKenney JK, Lohse CM, Leibovich BC, Thompson RH, Boorjian SA, et al. A novel grading system for clear cell renal cell carcinoma incorporating tumor necrosis. Am J Surg Pathol. 2013;37:311–22. https://doi.org/10.1097/PAS.0b013e318270f71c.
Khor LY, Dhakal HP, Jia X, Reynolds JP, McKenney JK, Rini BI, et al. Tumor necrosis adds prognostically significant information to grade in clear cell renal cell carcinoma: a study of 842 consecutive cases from a single institution. Am J Surg Pathol. 2016;40:1224–31. https://doi.org/10.1097/pas.0000000000000690.
Katz MD, Serrano MF, Grubb RL 3rd, Skolarus TA, Gao F, Humphrey PA, et al. Percent microscopic tumor necrosis and survival after curative surgery for renal cell carcinoma. J Urol. 2010;183:909–14. https://doi.org/10.1016/j.juro.2009.11.010.
Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–20. https://doi.org/10.1002/cncr.21206.
de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–84. https://doi.org/10.1097/00000478-200103000-00001.
Pichler R, Comperat E, Klatte T, Pichler M, Loidl W, Lusuardi L, et al. Renal cell carcinoma with sarcomatoid features: finally new therapeutic hope? Cancers. 2019;11. https://doi.org/10.3390/cancers11030422.
Przybycin CG, McKenney JK, Reynolds JP, Campbell S, Zhou M, Karafa MT, et al. Rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma: a clinicopathologic analysis of 76 cases with clinical follow-up. Am J Surg Pathol. 2014;38:1260–5. https://doi.org/10.1097/pas.0000000000000251.
Zhang BY, Cheville JC, Thompson RH, Lohse CM, Boorjian SA, Leibovich BC, et al. Impact of rhabdoid differentiation on prognosis for patients with grade 4 renal cell carcinoma. Eur Urol. 2015;68:5–7. https://doi.org/10.1016/j.eururo.2015.01.002.
Backhaus P, Noto B, Avramovic N, Grubert LS, Huss S, Bogemann M, et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45:860–77. https://doi.org/10.1007/s00259-017-3922-y.
Salas Fragomeni RA, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med. 2018;59:871–7. https://doi.org/10.2967/jnumed.117.203570.
Baccala A, Sercia L, Li J, Heston W, Zhou M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70:385–90. https://doi.org/10.1016/j.urology.2007.03.025.
Spatz S, Tolkach Y, Jung K, Stephan C, Busch J, Ralla B, et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol. 2018;199:370–7. https://doi.org/10.1016/j.juro.2017.08.079.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35. https://doi.org/10.1038/nrurol.2016.26.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2019.01.049.
Evangelista L, Basso U, Maruzzo M, Novara G. The role of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for the evaluation of renal cancer. Eur Urol Focus. 2020;6:146–50. https://doi.org/10.1016/j.euf.2018.08.004.
Ahn T, Roberts MJ, Abduljabar A, Joshi A, Perera M, Rhee H, et al. A review of prostate-specific membrane antigen (PSMA) positron emission tomography (PET) in renal cell carcinoma (RCC). Mol Imaging Biol. 2019;21:799–807. https://doi.org/10.1007/s11307-018-01307-0.
Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012;53:4–11. https://doi.org/10.2967/jnumed.111.093443.
Demirci E, Ocak M, Kabasakal L, Decristoforo C, Talat Z, Halac M, et al. (68)Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:1461–2. https://doi.org/10.1007/s00259-014-2766-y.
Rhee H, Blazak J, Tham CM, Ng KL, Shepherd B, Lawson M, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6:76. https://doi.org/10.1186/s13550-016-0231-6.
Raveenthiran S, Esler R, Yaxley J, Kyle S. The use of (68)Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46:2280–8. https://doi.org/10.1007/s00259-019-04432-2.
Sawicki LM, Buchbender C, Boos J, Giessing M, Ermert J, Antke C, et al. Diagnostic potential of PET/CT using a (68)Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: initial experience. Eur J Nucl Med Mol Imaging. 2017;44:102–7. https://doi.org/10.1007/s00259-016-3360-2.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8.
Funding
This research was supported by the National Natural Science Foundation of China (ID: 81772710, 81972388), the Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Key Medical Discipline (Laboratory) (ZDXKB2016014) and China Postdoctoral Science Foundation (2019T120418 and 2018M640477).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Gao, J., Xu, Q., Fu, Y. et al. Comprehensive evaluation of 68Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 48, 561–569 (2021). https://doi.org/10.1007/s00259-020-04916-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04916-6