Abstract
Purpose
Although most deep learning (DL) studies have reported excellent classification accuracy, these studies usually target typical Alzheimer’s disease (AD) and normal cognition (NC) for which conventional visual assessment performs well. A clinically relevant issue is the selection of high-risk subjects who need active surveillance among equivocal cases. We validated the clinical feasibility of DL compared with visual rating or quantitative measurement for assessing the diagnosis and prognosis of subjects with equivocal amyloid scans.
Methods
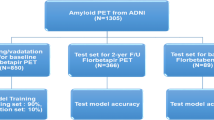
18F-florbetaben scans of 430 cases (85 NC, 233 mild cognitive impairment, and 112 AD) were assessed through visual rating-based, quantification-based, and DL-based methods. DL was trained using 280 two-dimensional PET images (80%) and tested by randomly assigning the remaining (70 cases, 20%) cases and a clinical validation set of 54 equivocal cases. In the equivocal cases, we assessed the agreement among the visual rating, quantification, and DL and compared the clinical outcome according to each modality-based amyloid status.
Results
The visual reading was positive in 175 cases, equivocal in 54 cases, and negative in 201 cases. The composite SUVR cutoff value was 1.32 (AUC 0.99). The subject-level performance of DL using the test set was 100%. Among the 54 equivocal cases, 37 cases were classified as positive (Eq(deep+)) by DL, 40 cases were classified by a second-round visual assessment, and 40 cases were classified by quantification. The DL- and quantification-based classifications showed good agreement (83%, κ = 0.59). The composite SUVRs differed between Eq(deep+) (1.47 [0.13]) and Eq(deep−) (1.29 [0.10]; P < 0.001). DL, but not the visual rating, showed a significant difference in the Mini-Mental Status Examination score change during the follow-up between Eq(deep+) (− 4.21 [0.57]) and Eq(deep−) (− 1.74 [0.76]; P = 0.023) (mean duration, 1.76 years).
Conclusions
In visually equivocal scans, DL was more related to quantification than to visual assessment, and the negative cases selected by DL showed no decline in cognitive outcome. DL is useful for clinical diagnosis and prognosis assessment in subjects with visually equivocal amyloid scans.






Similar content being viewed by others
References
Grundman M, Pontecorvo MJ, Salloway SP, Doraiswamy PM, Fleisher AS, Sadowsky CH, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27:4–15.
Hosokawa C, Ishii K, Hyodo T, Sakaguchi K, Usami K, Shimamoto K, et al. Investigation of (11)C-PiB equivocal PET findings. Ann Nucl Med. 2015;29:164–9.
Schreiber S, Landau SM, Fero A, Schreiber F, Jagust WJ. Alzheimer’s Disease Neuroimaging Initiative. Comparison of visual and quantitative florbetapir F 18 positron emission tomography analysis in predicting mild cognitive impairment outcomes. JAMA Neurol. 2015;72:1183–90.
Lecun Y, Bottou L, Bengio Y, Haffner P. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278–324.
Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10.
Liu M, Zhang D, Shen D. Alzheimer’s Disease Neuroimaging Initiative. Hierarchical fusion of features and classifier decisions for Alzheimer’s disease diagnosis. Hum Brain Mapp. 2014;35:1305–19.
Sarraf S, Tofighi G. Classification of Alzheimer’s disease structural MRI data by deep learning convolutional neural networks. 2017. https://arxiv.org/abs/1607.06583. Accessed 5 Apr 2019.
Wen D, Wei ZH, Zhou YH, Li GL, Zhang X, Han W. Deep learning methods to process fMRI data and their application in the diagnosis of cognitive impairment: a brief overview and our opinion. Front Neuroinform. 2018;12:23.
Singh S, Srivastava A, Mi L, Caselli RJ, Chen K, Goradia D, Reiman EM, Wang Y. Deep-learning-based classification of FDG-PET data for Alzheimer’s disease categories. In: 13th international conference on medical information processing and analysis. San Andres Islands, Colombia: International Society for Optics and Photonics; 2017. pp. 105720J.
Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57:1316–22.
NeuraCeq. NEURACEQ (florbetaben F 18 injection), highlights of prescribing information. 2017. http://piramal.com/neuraceq/images/Neuraceq_PI.pdf. Accessed 5 Apr 2019.
Van Maaten LD, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–605.
Vandenberghe R, Nelissen N, Salmon E, Ivanoiu A, Hasselbalch S, Andersen A, et al. Binary classification of 18F-flutemetamol PET using machine learning: comparison with visual reads and structural MRI. Neuroimage. 2013;64:517–25.
Ding Y, Sohn JH, Kawczynski MG, Trivedi H, Harnish R, Jenkins NW, et al. A deep learning model to predict a diagnosis of Alzheimer disease by using (18)F-FDG PET of the brain. Radiology. 2019;290:456–64.
Payoux P, Delrieu J, Gallini A, Adel D, Salabert AS, Hitzel A, et al. Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur J Nucl Med Mol Imaging. 2015;42:1459–68.
Cattell L, Platsch G, Pfeiffer R, Declerck J, Schnabel JA, Hutton C. Alzheimer’s Disease Neuroimaging Initiative. Classification of amyloid status using machine learning with histograms of oriented 3D gradients. Neuroimage Clin. 2016;12:990–1003.
Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–15.
Yuan Y, Wang Z, Lee W, VanGilder P, Chen Y, Reiman EM, et al. Quantification of amyloid burden from florbetapir pet images without using target and reference regions: preliminary findings based on the deep learning 3D convolutional neural network approach. Alzheimers Dement. 2018;14:P315–6.
Landau SM, Horng A, Jagust WJ. Initiative AsDN. Memory decline accompanies subthreshold amyloid accumulation. Neurology. 2018;90(17):e1452–e60.
La Joie R, Ayakta N, Seeley WW, Borys E, Boxer AL, DeCarli C, et al. Multisite study of the relationships between antemortem [11C] PIB-PET centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimers Dement. 2019;15(2):205–16.
Choi H, Jin KH. Alzheimer’s Disease Neuroimaging Initiative. Predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behav Brain Res. 2018;344:103–9.
Gamberger D, Lavrač N, Srivatsa S, Tanzi RE, Doraiswamy PM. Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Sci Rep. 2017;7:6763.
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–16.
Spiegel R, Berres M, Miserez AR, Monsch AU, Alzheimer’s Disease Neuroimaging Initiative. For debate: substituting placebo controls in long-term Alzheimer’s prevention trials. Alzheimers Res Ther. 2011;3:9.
Payan A, Montana G. Predicting Alzheimer’s disease: a neuroimaging study with 3D convolutional neural networks. 2015. https://arxiv.org/abs/1502.02506. Accessed 5 Apr 2019.
Yan K, Bagheri M, Summers RM. 3D context enhanced region-based convolutional neural network for end-to-end lesion detection. In: 21th international conference on medical image computing and computer assisted intervention. Granada, Spain: Springer; 2018. pp. 511–9.
Zhao G, Liu F, Oler JA, Meyerand ME, Kalin NH, Birn RM. Bayesian convolutional neural network based MRI brain extraction on nonhuman primates. Neuroimage. 2018;175:32–44.
Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78:439–53.
Funding
This study was supported by a grant from the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HI14C2768, HI14C3319); the Industrial Core Technology Development Program (10060305) funded by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Evaluation Institute of Industrial Technology (KEIT); the Asan Institute for Life Sciences (2014-0783, 2016-0588); and the Ministry of Science and ICT (MIST), Republic of Korea (2017M2A2A6A02020353).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the principles of the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology.
Hye Joo Son and Jungsu S.Oh contributed equally to this work.
Rights and permissions
About this article
Cite this article
Son, H.J., Oh, J.S., Oh, M. et al. The clinical feasibility of deep learning-based classification of amyloid PET images in visually equivocal cases. Eur J Nucl Med Mol Imaging 47, 332–341 (2020). https://doi.org/10.1007/s00259-019-04595-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04595-y