Abstract
Purpose
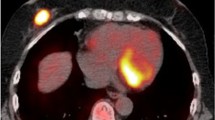
The aim of this study was to determine if functional parameters extracted from the hybrid positron emission tomography/magnetic resonance imaging (PET/MRI) correlate with the immunohistochemical markers of breast cancer (BC) lesions, to assess their ability to predict BC subtype.
Methods
This prospective study was approved by the institution’s Ethics Committee, and all patients provided written informed consent. A total of 50 BC patients at diagnosis underwent PET/MRI before pharmacological and surgical treatment. For each primary lesion, the following data were extracted: morphological data including tumour-node-metastasis stage and lesion size; apparent diffusion coefficient (ADC); perfusion data including forward volume transfer constant (Ktrans), reverse efflux volume transfer constant (Kep) and extravascular extracellular space volume (Ve); and metabolic data including standardized uptake value (SUV), lean body mass (SUL), metabolic tumour volume and total lesion glycolysis. Immunohistochemical reports were used to determine receptor status (oestrogen, progesterone, and human epidermal growth factor receptor 2), cellular differentiation status (grade), and proliferation index (Ki67) of the tumour lesions. Correlation studies (Mann–Whitney U test and Spearman’s test), receiver operating characteristic (ROC) curve analysis, and multivariate analysis were performed.
Results
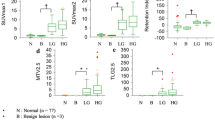
Association studies were performed to assess the correlations between imaging and histological prognostic markers of BC. Imaging biomarkers, which significantly correlated with biological markers, were selected to perform ROC curve analysis to determine their ability to discriminate among BC subtypes. SUVmax, SUVmean and SUL were able to discriminate between luminal A and luminal B subtypes (AUCSUVmean = 0.799; AUCSUVmax = 0.833; AUCSUL = 0.813) and between luminal A and nonluminal subtypes (AUCSUVmean = 0.926; AUCSUVmax = 0.917; AUCSUL = 0.945), and the lowest SUV and SUL values were associated with the luminal A subtype. Kepmax was able to discriminate between luminal A and luminal B subtypes (AUC = 0.779), and its highest values were associated with the luminal B subtype. Ktransmax (AUC = 0.881) was able to discriminate between luminal A and nonluminal subtypes, and the highest perfusion values were associated with the nonluminal subtype. In addition, ADC (AUC = 0.877) was able to discriminate between luminal B and nonluminal subtypes, and the lowest ADCmean values were associated with the luminal B subtype. Multivariate analysis was performed to develop a prognostic model, and the best predictive model included Ktransmax and SUVmax parameters.
Conclusion
Using multivariate analysis of both PET and MRI parameters, a prognostic model including Ktransmax and SUVmax was able to predict the tumour subtype in 38 of 49 patients (77.6%, p < 0.001), with higher accuracy for the luminal B subtype (86.2%).






Similar content being viewed by others
References
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23. https://doi.org/10.1093/annonc/mdt303.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using F-18-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (F-18-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8. https://doi.org/10.1093/jjco/hyn019.
Osborne JR, Port E, Gonen M, Doane AS, Yeung H, Gerald W, et al. F-18-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50. https://doi.org/10.2967/jnumed.108.060459.
Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93. https://doi.org/10.1007/s10147-010-0120-3.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high F-18-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35. https://doi.org/10.1007/s00259-010-1640-9.
Wang CL, MacDonald LR, Rogers JV, Aravkin A, Haseley DR, Beatty JD. Positron emission mammography: correlation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status and F-18-FDG. AJR Am J Roentgenol. 2011;197:W247–55. https://doi.org/10.2214/ajr.11.6478.
Koolen BB, Peeters M, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–8. https://doi.org/10.1007/s00259-012-2211-z.
Koo HR, Park JS, Kang KW, Cho N, Chang JM, Bae MS, et al. F-18-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24:610–8. https://doi.org/10.1007/s00330-013-3037-1.
Vicente AMG, Castrejon AS, Martin AL, Lopez-Muniz IC, Madero VM, Sanchez MDM, et al. Molecular subtypes of breast cancer: metabolic correlation with F-18-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11. https://doi.org/10.1007/s00259-013-2418-7.
Kitajima K, Fukushima K, Miyoshi Y, Nishimukai A, Hirota S, Igarashi Y, et al. Association between F-18-FDG uptake and molecular subtype of breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:1371–7. https://doi.org/10.1007/s00259-015-3070-1.
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, et al. Utility of F-18 FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209–17. https://doi.org/10.1007/s10549-015-3303-9.
Chen JH, Baek HM, Nalcioglu O, Su MY. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging. 2008;27:825–33. https://doi.org/10.1002/jmri.21330.
Cipolla V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, de Felice C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur J Radiol. 2014;83:2144–50. https://doi.org/10.1016/j.ejrad.2014.09.015.
Kim JY, Kim SH, Kim YJ, Kang BJ, An YY, Lee AW, et al. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging. 2015;33:72–80. https://doi.org/10.1016/j.mri.2014.08.034.
Mori N, Ota H, Mugikura S, Takasawa C, Ishida T, Watanabe G, et al. Luminal-type breast cancer: correlation of apparent diffusion coefficients with the Ki-67 labeling index. Radiology. 2015;274:66–73. https://doi.org/10.1148/radiol.14140283.
Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 tesla. J Magn Reson Imaging. 2015;41:175–82. https://doi.org/10.1002/jmri.24519.
Sharma U, Sah RG, Agarwal K, Parshad R, Seenu V, Mathur SR, et al. Potential of diffusion-weighted imaging in the characterization of malignant, benign, and healthy breast tissues and molecular subtypes of breast cancer. Front Oncol. 2016;6:126. https://doi.org/10.3389/fonc.2016.00126.
Shin HJ, Park JY, Shin KC, Kim HH, Cha JH, Chae EY, et al. Characterization of tumor and adjacent peritumoral stroma in patients with breast cancer using high-resolution diffusion-weighted imaging: correlation with pathologic biomarkers. Eur J Radiol. 2016;85:1004–11. https://doi.org/10.1016/j.ejrad.2016.02.017.
Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250:638–47. https://doi.org/10.1148/radiol.2503081054.
Shin JK, Kim JY. Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging. 2017;45:94–102. https://doi.org/10.1002/jmri.25348.
Wu MX, Ma J. Association between imaging characteristics and different molecular subtypes of breast cancer. Acad Radiol. 2017;24:426–34. https://doi.org/10.1016/j.acra.2016.11.012.
Miyake KK, Nakamoto Y, Kanao S, Tanaka S, Sugie T, Mikami Y, et al. Diagnostic value of F-18-FDG PET/CT and MRI in predicting the clinicopathologic subtypes of invasive breast cancer. AJR Am J Roentgenol. 2014;203:272–9. https://doi.org/10.2214/ajr.13.11971.
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37:2011–20. https://doi.org/10.1007/s00259-010-1529-7.
Choi BB, Kim SH, Kang BJ, Lee JH, Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012;10:126. https://doi.org/10.1186/1477-7819-10-126.
Byun BH, Noh WC, Lim I, Lee SS, Cho AR, Park JA, et al. A new method for apparent diffusion coefficient measurement using sequential F-18-FDG PET and MRI: correlation with histological grade of invasive ductal carcinoma of the breast. Ann Nucl Med. 2013;27:720–8. https://doi.org/10.1007/s12149-013-0737-1.
Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in F-18-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55:736–42. https://doi.org/10.2967/jnumed.113.129395.
Kitajim K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016;85:943–9. https://doi.org/10.1016/j.ejrad.2016.02.015.
Catalano OA, Daye D, Signore A, Iannace C, Vangel M, Luongo A, et al. Staging performance of whole-body DWI, PET/CT and PET/MRI in invasive ductal carcinoma of the breast. Int J Oncol. 2017;51:281–8. https://doi.org/10.3892/ijo.2017.4012.
Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients – a hypothesis-generating exploratory study. Radiology. 2013;269:857–69. https://doi.org/10.1148/radiol.13131306.
Pace L, Nicolai E, Luongo A, Aiello M, Catalano OA, Soricelli A, et al. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol. 2014;83:289–96.
Wiesmuller M, Quick HH, Navalpakkam B, Lell MM, Uder M, Ritt P, et al. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:12–21. https://doi.org/10.1007/s00259-012-2249-y.
Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. https://doi.org/10.1007/s00259-009-1297-4.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410
Martinez-Moller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–6. https://doi.org/10.2967/jnumed.108.054726.
Yuan J, Chow SKK, Yeung DKW, Ahuja AT, King AD. Quantitative evaluation of dual-flip-angle T1 mapping on DCE-MRI kinetic parameter estimation in head and neck. Quant Imaging Med Surg. 2012;2:245–53. https://doi.org/10.3978/j.issn.2223-4292.2012.11.04.
Fritz-Hansen T, Rostrup E, Larsson HB, Sondergaard L, Ring P, Henriksen O. Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med. 1996;36:225–31. https://doi.org/10.1002/mrm.1910360209.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. https://doi.org/10.1093/annonc/mdr304.
Plecha DM, Faulhaber P. PET/MRI of the breast. Eur J Radiol. 2017;94:A26–34. https://doi.org/10.1016/j.ejrad.2017.05.006.
Goorts B, Voo S, van Nijnatten TJA, Kooreman LFS, de Boer M, Keymeulen KBMI, et al. Hybrid F-18-FDG PET/MRI might improve locoregional staging of breast cancer patients prior to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2017;44:1796–805. https://doi.org/10.1007/s00259-017-3745-x.
Cho N, Im SA, Cheon GJ, Park IA, Lee KH, Kim TY, et al. Integrated 18F-FDG PET/MRI in breast cancer: early prediction of response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45(3):328–39. https://doi.org/10.1007/s00259-017-3849-3.
Catalano OA, Horn GL, Signore A, Iannace C, Lepore M, Vangel M, et al. PET/MR in invasive ductal breast cancer: correlation between imaging markers and histological phenotype. Br J Cancer. 2017;116:893–902. https://doi.org/10.1038/bjc.2017.26.
Higuchi T, Nishimukai A, Ozawa H, Fujimoto Y, Yanai A, Miyagawa Y, et al. Prognostic significance of preoperative F-18-FDG PET/CT for breast cancer subtypes. Breast. 2016;30:5–12. https://doi.org/10.1016/j.breast.2016.08.003.
Yoon HJ, Kang KW, Chun IK, Cho N, Im SA, Jeong S, et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from Ga-68-RGD PET/CT and F-18-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:1534–43. https://doi.org/10.1007/s00259-014-2744-4.
De Cicco C, Gilardi L, Botteri E, Fracassi SLV, Di Dia GA, Botta F, et al. Is F-18 fluorodeoxyglucose uptake by the primary tumor a prognostic factor in breast cancer? Breast. 2013;22:39–43. https://doi.org/10.1016/j.breast.2012.05.009.
Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol. 2012;85:e474–9. https://doi.org/10.1259/bjr/79381464.
Lee HS, Kim SH, Kang BJ, Baek JE, Song BJ. Perfusion parameters in dynamic contrast-enhanced MRI and apparent diffusion coefficient value in diffusion-weighted MRI: association with prognostic factors in breast cancer. Acad Radiol. 2016;23:446–56. https://doi.org/10.1016/j.acra.2015.12.011.
Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011;33:102–9. https://doi.org/10.1002/jmri.22400.
Molinari C, Clauser P, Girometti R, Linda A, Cimino E, Puglisi F, et al. MR mammography using diffusion-weighted imaging in evaluating breast cancer: a correlation with proliferation index. Radiol Med. 2015;120:911–8. https://doi.org/10.1007/s11547-015-0527-z.
Koo HR, Cho N, Song IC, Kim H, Chang JM, Yi A, et al. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging. 2012;36:145–51. https://doi.org/10.1002/jmri.23635.
Youk JH, Son EJ, Chung J, Kim JA, Kim EK. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol. 2012;22:1724–34. https://doi.org/10.1007/s00330-012-2425-2.
Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, et al. Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. 2017;18(4). https://doi.org/10.3390/ijms18040805.
Monti S, Aiello M, Incoronato M, Grimaldi AM, Moscarino M, Mirabelli P, et al. DCE-MRI pharmacokinetic-based phenotyping of invasive ductal carcinoma: a radiomic study for prediction of histological outcomes. Contrast Media Mol Imaging. 2018;2018:5076269. https://doi.org/10.1155/2018/5076269
Mottaghy FM. Is the whole larger than the sum of the parts? Integrated PET/MRI as a tool for response prediction. Eur J Nucl Med Mol Imaging. 2018;45:325–7. https://doi.org/10.1007/s00259-017-3908-9
Funding
This study was supported by “Ricerca Corrente” funding from the Italian Ministry of Health and partially by “5 per mille” IRCCS SDN grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the institutional Ethics Committee (Prot2-11, IRCCS Fondazione SDN). This article does not describe any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Incoronato, M., Grimaldi, A.M., Cavaliere, C. et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: a PET/MRI study. Eur J Nucl Med Mol Imaging 45, 1680–1693 (2018). https://doi.org/10.1007/s00259-018-4010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4010-7