Abstract
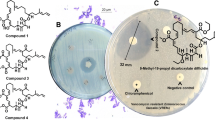
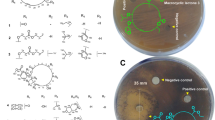
Intertidal red algae Hypnea valentiae associated Bacillus amyloliquefaciens MTCC 12716 revealed potential inhibitory effects on the growth of drug-resistant pathogens. In the genome of B. amyloliquefaciens MTCC 12716, biosynthetic gene clusters encoding antibacterial metabolites were predicted, which might be expressed and contributed to the broad-spectrum anti-infective activity. Three homologue members of the 24-membered macrocyclic lactone family, named as bacvalactones 1–3 bearing 13-O-ethyl (1); 15-O-furanyl-13-O-isobutyl-7-O-propyl-propanoate (2); and 15-O-furanyl-13-O-isobutyl-7-O-propyl-propanoate-7,24-dimethyl (3) functionalities, were acquired through bioactivity-guided purification. The macrocyclic lactones displayed bactericidal activity against opportunistic pathogens causing nosocomial infections, for instance, methicillin–resistant Staphylococcus aureus (MRSA), vancomycin–resistant Enterococcus faecalis (VREfs), and multidrug-resistant strains of Pseudomonas aeruginosa and Klebsiella pneumonia with MIC ≤ 3.0 μg/mL, whereas standard antibiotics ampicillin and chloramphenicol were active only at concentrations of ≥ 6.25 mg/mL. The biosynthetic pathway of macrocyclic lactones that are generated by trans-AT polyketide synthases through stepwise extension of an acetyl starter unit by eleven sequential Claisen condensations with malonyl-CoA was established, and the structures were correlated with the gene organization of the mln operon, which encompasses nine genes mln A-I (approximately 47 kb in size). The best binding poses for each compounds (1–3) with Staphylococcus aureus peptide deformylase (SaPDF) unveiled docking scores (≥ 9.70 kcal/mol) greater than that of natural peptide deformylase inhibitors, macrolactin N and actinonin (9.14 and 6.96 kcal/mol, respectively), which supported their potential in vitro bioactivities. Thus, the present work demonstrated the potential of macrocyclic lactone for biotechnological and pharmaceutical applications against emerging multidrug-resistant pathogens.
Key Points
•Three antibacterial bacvalactones were identified from the symbiotic bacterium.
•The symbiotic bacterial genome was explored to identify the biosynthetic gene clusters.
•Trans-AT pks-assisted mln biosynthetic pathway of the macrocyclic lactone was proposed.
•In silico molecular interactions of the bacvalactones with S. aureus PDF were analyzed.






Similar content being viewed by others
References
Aleti G, Sessitsch A, Brader G (2015) Genome mining: prediction of lipopeptides and polyketides from Bacillus and related Firmicutes. Comput Struct Biotechnol J 13:192–203
Anjum K, Abbas SQ, Ali Shah SA, Akhter N, Batool S, ul Hassan SS (2016) Marine sponges as a drug treasure. Biomol Ther 24:347–362
Arias CA, Murray BE (2009) Antibiotic-resistant bugs in the 21st century-a clinical super-challenge. New Engl J Med 360(5):439–443
Armstrong E, Rogerson A, Leftley JW (2000) The abundance of heterotrophic protists associated with intertidal seaweeds. Estuar Coast Shelf Sci 50:415–424
Avendaño-Herrera R, Lody M, Riquelme CE (2005) Produccion de substancias inhibitorias entre bacterias de biopeliculas en substratos marinos. Revista de Biologia Marina y Oceanografía 40(2):117–125
Bengtsson M, Sjøtun K, Øvreås L (2010) Seasonal dynamics of bacterial biofilms on kelp (Laminaria hyperborea). Aquat Microb Ecol 60:71–83
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T (2019) antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87
Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR (2016) Marine natural products. Nat Prod Rep 33:382–431
Chakraborty K, Thilakan B, Raola VK (2014) Polyketide family of novel antibacterial 7-O-methyl-5′-hydroxyl 3′-heptenoate-macrolactin from seaweed associated Bacillus subtilis MTCC 10403. J Agric Food Chem 62:12194–12208
Chakraborty K, Thilakan B, Raola VK (2017) Antimicrobial polyketide furanoterpenoids from seaweed associated heterotrophic bacterium Bacillus subtilis MTCC 10403. Phytochemistry 142:112–125
Chakraborty K, Thilakan B, Kizhakkekalm VK (2018) Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. J Appl Microbiol 124:108–125
Chen D, Yuan Z (2005) Therapeutic potential of peptide deformylase inhibitors. Expert Opin Inv Drug 14(9):1107–1116
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R, Borriss R (2009) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
DeLeo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375(9725):1557–1568
Gao CH, Tian XP, Qi SH, Luo XM, Wang P, Zhang S (2010) Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J Antibiot 63(4):191–193
Giglione C, Pierre M, Meinnel T (2000) Peptide deformylase as a target for new generation, broad spectrum antimicrobial agents. Mol Microbiol 36(6):1197–1205
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–300
Gomez VLJ, Soria-Mercado IE, Rivas GG, Ayala-Sanchez NE (2010) Antibacterial and anticancer activity of seaweeds and bacteria associated with their surface. Rev Biol Mar Oceanogr 45:267–275
Guay DRP (2007) Drug forecast-the peptide deformylase inhibitors as antibacterial agents. Ther Clin Risk Manag 4:513–525
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429
Harwood CR, Mouillon JM, Pohl S, Arnau J (2018) Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev 42(6):721–738
Helaly SE, Thongbai B, Stadler M (2018) Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat Prod Rep 35(9):992–1014
Kijjoa A, Sawangwong P (2004) Drugs and cosmetics from the sea. Mar Drugs 2(2):73–82
Kizhakkekalam VK, Chakraborty K (2019) Pharmacological properties of marine macroalgae-associated heterotrophic bacteria. Arch Microbiol 201:505–518
Kizhakkekalam VK, Chakraborty K (2020) Marine macroalgae-associated heterotrophic Firmicutes and Gamma-proteobacteria: prospective anti-infective agents against multidrug resistant pathogens. Arch Microbiol 202:905–920.
Kizhakkekalam VK, Chakraborty K, Joy M (2020) Oxygenated elansolid type of polyketide spanned macrolides from a marine heterotrophic Bacillus as prospective antimicrobial agents against multidrug resistant pathogens. Int J of Antimicrob Agents 55(3):105892.
Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL (2002) Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8(2):160–166
Lachnit T, Meske D, Wahl M, Harder T, Schmitz R (2011) Epibacterial community patterns on marine macroalgae are host-specific but temporally variable. Environ Microbiol 13:655–665
Lee S-J, Cho J-Y, Cho J-I, Moon J-H, Park KD, Lee YJ, Park K-H (2004) Isolation and characterization of antimicrobial substance macrolactin A produced from Bacillus amyloliquefaciens CHO104 isolated from soil. J Microbiol Biotechn 14:525–531
Li JW, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165
Lu XL, Xu QZ, Liu XY, Cao X, Ni KY, Jiao BH (2008) Marine drugs-macrolactins. Chem Biodivers 5:1669–1674
Miesel L, Greene J, Black TA (2003) Genetic strategies for antibacterial drug discovery. Nat Rev Genet 4:442–456
Mondol MA, Kim JH, Lee HS, Lee YJ, Shin HJ (2011) Macrolactin W, a new antibacterial macrolide from a marine Bacillus sp. Bioorg Med Chem Lett 21(12):3832–3835
Mondol MA, Shahidullah Tareq F, Kim JH, Lee MA, Lee HS, Lee JS, Lee YJ, Shin HJ (2013) New antimicrobial compounds from a marine-derived Bacillus sp. J Antibiot 66:89–95
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Murray BE (2000) Vancomycin-resistant enterococcal infections. N Engl J Med 342(10):710–721
Nagao T, Adachi K, Sakai M, Nishijima M, Sano H (2001) Novel macrolactins as antibiotic lactones from a marine bacterium. J Antibiot 54:333–339
Pedersen PB, Bjørnvad ME, Rasmussen MD, Petersen JN (2002) Cytotoxic potential of industrial strains of Bacillus sp. Regul Toxicol Pharmacol 36:155–161
Piel J (2010) Biosynthesis of polyketides by trans-AT polyketide synthases. Nat Prod Rep 27:996–1047
Rychnovsky SD, Skalitzky DJ, Pathirana C, Jensen PR, Fenical W (1992) Stereochemistry of the macrolactins. J Am Chem Soc 114:671–677
Schneider K, Chen XH, Vater J, Franke P, Nicholson G, Borriss R, Süssmuth RD (2007) Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J Nat Prod 70:1417–1423
Yoo JS, Zheng CJ, Lee S, Kwak JH, Kim WG (2006) Macrolactin N, a new peptide deformylase inhibitor produced by Bacillus subtilis. Bioorg Med Chem Lett 16:4889–92.
Yoon HJ, Kim HL, Lee SK, Kim HW, Kim HW, Lee JY, Mikami B, Suh SW (2004) Crystal structure of peptide deformylase from Staphylococcus aureus in complex with actinonin, a naturally occurring antibacterial agent. Proteins 57(3):639–642
Zheng CJ, Lee S, Lee CH, Kim WG (2007) Macrolactins O-R, glycosylated 24-membered lactones from Bacillus sp. AH159-1. J Nat Prod 70(10):1632–1635
Acknowledgments
The authors are thankful to the Indian Council of Agricultural Research (ICAR), New Delhi, for providing facilities to carry out the work. The authors thank the Director of Central Marine Fisheries Research Institute and Dean, Faculty of Marine Sciences, Lakeside Campus, Cochin University of Science and Technology, for support. Thanks are due to the Head of Marine Biotechnology Division, Central Marine Fisheries Research Institute, for facilitating research activities.
Funding
This work was supported by funding under the Kerala State Council for Science, Technology and Environment (grant number 040/FSHP-LSS/2014/KSCSTE) and Science and Engineering Research Board (SERB) Scheme (grant number SR/S1/OC-96A/2012) from the Department of Science and Technology, New Delhi, India.
Author information
Authors and Affiliations
Contributions
KC and VKK conceived and designed research, acquired funds, and conducted experiments. MJ analyzed data. RDC drafted the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 6624 kb)
Rights and permissions
About this article
Cite this article
Chakraborty, K., Kizhakkekalam, V.K., Joy, M. et al. Moving away from traditional antibiotic treatment: can macrocyclic lactones from marine macroalga-associated heterotroph be the alternatives?. Appl Microbiol Biotechnol 104, 7117–7130 (2020). https://doi.org/10.1007/s00253-020-10658-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10658-0