Abstract
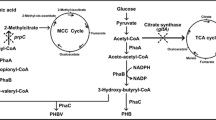
Microbial polyhydroxyalkanoates (PHA) are a family of biopolyesters with properties similar to petroleum plastics such as polyethylene (PE) or polypropylene (PP). Polyhydroxybutyrate (PHB) is the most common PHA known so far. Clustered regularly interspaced short palindromic repeats interference (CRISPRi), a technology recently developed to control gene expression levels in eukaryotic and prokaryotic genomes, was employed to regulate PHB synthase activity influencing PHB synthesis. Recombinant Escherichia coli harboring an operon of three PHB synthesis genes phaCAB cloned from Ralstonia eutropha, was transformed with various single guided RNA (sgRNA with its guide sequence of 20–23 bases) able to bind to various locations of the PHB synthase PhaC, respectively. Depending on the binding location and the number of sgRNA on phaC, CRISPRi was able to control the phaC transcription and thus PhaC activity. It was found that PHB content, molecular weight, and polydispersity were approximately in direct and reverse proportion to the PhaC activity, respectively. The higher the PhaC activity, the more the intracellular PHB accumulation, yet the less the PHB molecular weights and the wider the polydispersity. This study allowed the PHB contents to be controlled in the ranges of 1.47–75.21% cell dry weights, molecular weights from 2 to 6 millions Dalton and polydispersity of 1.2 to 1.43 in 48 h shake flask studies. This result will be very important for future development of ultrahigh molecular weight PHA useful to meet high strength application requirements.

Similar content being viewed by others
References
Andreesen B, Steinbüchel A (2010) Biosynthesis and biodegradation of 3-hydroxypropionate-containing polyesters. Appl Environ Microbiol 76:4919–4925. doi:10.1128/AEM.01015-10
Antonio RV, Steinbuchel A, Rehm BHA (2000) Analysis of in vivo substrate specificity of the PHA synthase from Ralstonia eutropha: formation of novel copolyesters in recombinant Escherichia coli. FEMS Microbiol Lett 182:111–117. doi:10.1111/j.1574-6968.2000.tb08883.x
Braunegg G, Sonnleitner B, Lafferty RM (1978) Rapid gas-chromatographic method for determination of poly-β-hydroxybutyric acid in microbial biomass. J Appl Microbiol Biotechnol 6:29–37. doi:10.1007/BF00500854
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446. doi:10.1039/B812677C
Chen GQ, Page WJ (1997) Production of poly-beta-hydroxybutyrate by Azotobacter vinelandii in a two-stage fermentation process. Biotechnol Tech 11:347–350. doi:10.1023/A:1018435815864
Chylinski K, Makarova KS, Charpentier E, Koonin EV (2014) Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res 42:6091–6105. doi:10.1093/nar/gku241
Choi JI, Lee SY (2004) High level production of supra molecular weight poly(3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol Bioprocess Eng 9:196–200. doi:10.1007/BF02942292
Cong L, Ran F, Cox D, Lin S, Barretto R, Habi N, Hsu P, Wu X, Marraffini L, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819−823. doi:10.1126/ science. 1231143
Doi Y, Kunioka M, Nakamura Y, Soga K (1988) Nuclear magnetic-resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate. Macromolecules 21:2722–2727
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826. doi:10.1038/nbt.2623
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci 109:2579–2586. doi:10.1073/pnas.1208507109
Hiroe A, Hyakutake M, Thomson NM, Sivaniah E, Tsuge T (2013a) Endogenous ethanol affects biopolyester molecular weight in recombinant Escherichia coli. ACS Chem Biol 8:2568–2576. doi:10.1021/cb400465p
Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T (2012) Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly[(R)-3-Hydroxybutyrate] in genetically engineered Escherichia coli. Appl Environ Microbiol 78:3177–3184. doi:10.1128/AEM.07715-11
Hiroe A, Ushimaru K, Tsuge T (2013b) Characterization of polyhydroxyalkanoate (PHA) synthase derived from Delftia acidovorans DS-17 and the influence of PHA production in Escherichia coli. J Biosci Bioeng 115:633–638. doi:10.1016/j.jbiosc.2012.12.015
Hyakutake M, Tomizawa S, Mizuno K, Abe H, Tsuge T (2014) Alcoholytic cleavage of polyhydroxyalkanoate chains by class IV synthases induced by endogenous and exogenous ethanol. Appl Environ Microbiol 80:1421–1429. doi:10.1128/AEM.03576-13
Jendrossek D, Pfeiffer D (2014) New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 16:2357–2373. doi:10.1111/1462-2920.12356
Kusaka S, Abe H, Lee SY, Doi Y (1997) Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl Microbiol Biotechnol 47:140–143. doi:10.1007/s002530050902
Lee SY, Middelberg APJ, Lee YK (1997) Poly(3-hydroxybutyrate) production from whey using recombinant Escherichia coli. Biotechnol Lett 19:1033–1035. doi:10.1023/A:1018411820580
Li YF, Lin ZQ, Huang C, Zhang Y, Wang ZW, Tang YJ, Chen T, Zhao XM (2015) Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab Eng 31:13–21. doi:10.1016/j.ymben.2015.06.006
Li ZJ, Shi ZY, Jian J, Guo YY, Wu Q, Chen GQ (2010) Production of poly(3 hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng 12:352–359. doi:10.1016/j.ymben.2010.03.003
Lv L, Ren YL, Chen JC, Wu Q, Chen GQ (2015) Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study:controllable P (3HB-co-4HB) biosynthesis. Metab Eng 29:160–168. doi:10.1016/j.ymben.2015.03.013
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826. doi:10.1126/science.1232033
Meng DC, Shi ZY, Wu LP, Zhou Q, Wu Q, Chen JC, Chen GQ (2012) Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab Eng 14:317–324. doi:10.1016/j.ymben.2012.04.003
Nakamura S, Kunioka M, Doi, Y (1991) Biosynthesis and characterization of bacterial poly(3-hydroxybutyrate-co-3-hydroxypropionate). J Macromol Sci Chem A 28:15–24. doi:10.1080/00222339108054378
Nomura CT, Taguchi S (2007) PHA synthase engineering toward super biocatalysts for custom-made biopolymers. Appl Microbiol Biotechnol 73:969–979. doi:10.1007/s00253-006-0566-4
Ouyang SP, Liu Q, Fang L, Chen GQ (2007) Construction of pha-Operon-Defined Knockout Mutants of Pseudomonas putida KT2442 and their Applications in Poly(hydroxyalkanoate) Production. Macromol Biosci 7:227–233. doi:10.1002/mabi.200600187
Park SJ, Kim TW, Kim MK, Lee SY, Lim SC (2012) Advanced bacterial polyhydroxyalkanoates: towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol Adv 30:1196–1206. doi:10.1016/j.biotechadv.2011.11.007
Peoples OP, Sinskey AJ (1989) Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes-eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J Biol Chem 264:15298–15303
Qi L, Larson M, Gilbert L, Doudna J, Weissman J, Arkin A, Lim W (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. doi:10.1016/j.cell.2013.02.022
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33. doi:10.1042/BJ20031254
Silva LF, Gomez JGC, Oliveira MS, Torres BB (2000) Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. Journal of biotechnology.76:165–174. doi:10.1016/S0168-1656(99)00184-4
Steinbüchel A (2001) Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol Biosci 1:1–24. doi:10.1002/1616-5195(200101)1:1<1::AID-MABI1>3.0.CO;2-B
Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H (1992) Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev 9:217–230
Steinbüchel A, Schlegel HG (1991) Physiology and molecular-genetics of poly(beta hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol 5:535–542. doi:10.1111/j.1365-2958.1991.tb00725.x
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228. doi:10.1111/j.1574-6968.1995.tb07528.x
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:503–1555. doi:10.1016/S0079-6700(00)00035-6
Thomson NM, Hiroe A, Tsuge T, Summers DK, Sivaniah E (2014) Efficient molecular weight control of bacterially synthesized polyesters by alcohol supplementation. J Chem Technol Biotechnol 89:1110–1114. doi:10.1002/jctb.4198
Tomizawa S, Hyakutake M, Saito Y, Agus J, Mizuno, K, Abe H, Tsuge T (2011) Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 12: 2660–2666. doi:10.1021/bm2004687
Tomizawa S, Sato S, Lan JCW, Nakamura Y, Abe H, Tsuge T (2013) In vitro evidence of chain transfer to tetraethylene glycols in enzymatic polymerization of polyhydroxyalkanoate. Appl Microbiol Biot 97:4821–4829. doi:10.1007/s00253-013-4798-9
Tsuge T, Saito Y, Narike M, Muneta K, Normi YM, Kikkawa Y, Hiraishi T, Doi Y (2004) Mutation effects of a conserved alanine (Ala510) in type I polyhydroxyalkanoate synthase from Ralstonia eutropha on polyester biosynthesis. Macromol Biosci 4:963–970. doi:10.1002/mabi.200400075
Tyo KEJ, Fischer CR, Simeon F, Stephanopoulos G (2010) Analysis of polyhydroxybutyrate flux limitations by systematic genetic and metabolic perturbations. Metab Eng 12:187–195. doi:10.1016/j.ymben.2009.10.005
Valentin HE, Mitsky TA, Mahadeo DA, Tran M, Gruys KJ (2000) Application of a propionyl coenzyme A synthetase for poly(3-hydroxypropionate-co-3-hydroxybutyrate) accumulation in recombinant Escherichia coli. Appl Environ Microb 6:5253–5258. doi:10.1128/AEM.66.12.5253-5258.2000
Wang HH, Zhou XR, Liu Q, Chen GQ (2011) Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl Microbiol Biotechnol 89:1497–1507. doi:10.1007/s00253-010-2964-x
Wang L, Wang ZH, Shen CY, You ML, Xiao JF, Chen GQ (2010) Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 31:1691–1698. doi:10.1016/j.biomaterials.2009.11.053
Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482:331–338. doi:10.1038/nature10886
Wodzinska J, Snell KD, Rhomberg A, Sinskey AJ, Biemann K, Stubbe J (1996) Polyhydroxybutyrate synthase: evidence for covalent catalysis. J Am Chem Soc 26:6319–6320. doi:10.1021/ja961108a
Yao YC, Zhan XY, Zhang J, Zou XH, Wang ZH, Xiong YC, Chen JC, Chen GQ (2008) A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials 29:4823–4830. doi:10.1016/j.biomaterials.2008.09.008
Yin J, Wang H, Fu XZ, Gao X, Wu Q, Chen GQ (2015) Effects of chromosomal gene copy number and locations on polyhydroxyalkanoate synthesis by Escherichia coli and Halomonas sp. Appl Microbiol Biotechnol 99:5523–5534. doi:10.1007/s00253-015-6510-8
You ML, Peng GF, Li J (2011) Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials 32:2305–2313. doi:10.1016/j.biomaterials.2010.12.009
Acknowledgements
Plasmid pBHR68 was kindly donated by Professor Alexander Steinbüchel of Munster University in Germany. This study was funded by the State Key Research Project (Grant no. 2016YFB0302504) and National Natural Science Foundation of China (Grant no. 31430003 and 31270146). A special Tsinghua Presidential project (Grant no. 2015THZ10) also contributed to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, D., Lv, L., Chen, JC. et al. Controlling microbial PHB synthesis via CRISPRi. Appl Microbiol Biotechnol 101, 5861–5867 (2017). https://doi.org/10.1007/s00253-017-8374-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8374-6