Abstract
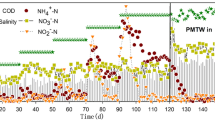
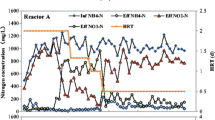
In this study, a non-woven rotating biological contactor reactor was operated for the start-up of completely autotrophic nitrogen removal over nitrite (CANON) process. In this perfectly attached growth system, nitrite oxidizing was identified, which interfered with the nitrogen removal performance. Batch tests indicated that 10 g NaCl per liter salinity was a preferable definite level to stand out ammonium-oxidizing activity and anammox activity, and selectively suppress nitrite-oxidizing activity under oxygen-limited conditions. Reactor operation showed that the maximum TN removal rate was increased from 425 mg N l−1 day−1 to 637 mg N l−1 day−1 after the addition of 10 g NaCl per liter salinity on analogous technological parameters. Microbiological community analysis revealed that bacteria strains similar to the genus Nitrospira sp. were specialized nitrite oxidizers existing in CANON reactor, which were then eliminated under salinity exposure for their no salinity-tolerant relative. However, anammox bacteria belonging to Planctomycetes and some aerobic ammonium oxidizers belonging to Nitrosomonas could be highly enriched under this oxygen-limited salinity conditions. Salinity-contained high ammonium wastewater will be so considered as suitable influent for CANON process in further industrial application.




Similar content being viewed by others
References
Abeliovich A (1985) Nitrification of ammonia in wastewater: field observations and laboratory studies. Water Res 19:1097–1099
Abeliovich A (1987) Nitrifying bacteria in wastewater reservoirs. Appl Environ Microbiol 53:754–760
Altschul SF, Gish W, Miller W, Myers E, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
American Public Health Association (1995) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Boon N, De Windt W, Verstraete W, Top EM (2002) Evaluation of nested PCR-DGGE (denaturing gradient gel electrophoresis) with group-specific 16S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiol Ecol 39:101–112
Campos JL, Mosquera-Collal A, Sānchez M, Méndez R, Lema JM (2002) Nitrification in saline wastewater with high ammonia concentration in an activated sludge unit. Water Res 36:2555–2560
Catalan MAB, Wang PC, Matsumura M (1997) Nitrification performance of marine nitrifiers immobilized in polyester and macro-porous cellulose carriers. J Ferment Bioeng 84(6):563–571
Cébron A, Garnier J (2005) Nitrobacter and Nitrospira genera as representatives of nitrite-oxidizing bacteria: detection, quantification and growth along the lower Seine River (France). Water Res 39:4979–4992
Chuang H, Ohashi A, Imachi H, Tandukar M, Harada H (2007) Effective partial nitrification to nitrite by down-flow hanging sponge reactor under limited oxygen condition. Water Res 41:295–302
Colt J, Tomasso JR (2001) Hatchery water supply and treatment. In: Wedemeyer GA (ed) Fish hatchery management (seconded.). American Fisheries Society, Bethesda, Maryland, pp 91–186
Dabert P, Delgenes JP, Moletta R, Godon JJ (2002) Contribution of molecular microbiology to the study in water pollution removal of microbial community dynamic. Rev Environ Sci Biotechnol 1(1):39–49
Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M (2000) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Dincer AR, Kargi F (2001) Performance of rotating biological disc system treating saline wastewater. Process Biochem 36(8–9):901–906
Egli K, Bosshard F, Werlen C, Lais P, Siegrist H, Zehnder AJB, van der Meer JR (2003) Microbial composition and structure of a rotating biological contactor biofilm treating ammonium-rich wastewater without organic carbon. FEMS Microbiol Ecol 45:419–432
Fontenot Q, Bonvillain C, Kilgen M, Boopathy R (2007) Effects of temperature, salinity, and carbon: nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater. Bioresour Technol 98:1700–1703
Fujii T, Sugino H, Rouse JD, Furukawa K (2002) Characterization of the microbial community in an anaerobic ammonium-oxidizing biofilm cultured on a nonwoven biomass carrier. J Biosci Bioeng 94:412–418
Furukawa K, Rouse JD, Yoshida N, Hatanaka H (2003) Mass cultivation of anaerobic ammonium-oxidizing sludge using a novel nonwoven biomass carrier. J Chem Eng Jpn 36(10):1163–1169
Gieseke A, Purkhold U, Wagner M, Amann R, Schramm A (2001) Community structure and activity dynamics of nitrifying bacteria in a phosphate-removing biofilm. Appl Environ Microbiol 67:1351–1362
Gong Z, Liu, S, Yang, F, Bao H, Hu, S, Furukawa K (2008) Characterization of functional microbial community in a membrane-aerated biofilm reactor operated for completely autotrophic nitrogen removal. Bioresour Technol 99(8):2749–2756
Hao X, Heijnen JJ, van Loosdrecht MCM (2001) Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol Bioeng 77(3):266–277
Kartal B, Koleva M, Arsov R, van der Star W, Jetten MSM, Strous M (2006) Adaptation of a freshwater anammox population to high salinity wastewater. J Biotechnol 126(4):546–553
Kowalchuk GA, Bodelier PLE, Heilig GHJ, Stephen JR, Laanbroek HJ (1998) Community analysis of ammonia-oxidising bacteria, in relation to oxygen availability in soils and root-oxygenated sediments, using PCR, DGGE and oligonucleotide probe hybridisation. FEMS Microbiol Ecol 27:339–350
Lakay FM, Botha A, Prior BA (2007) Comparative analysis of environmental DNA extraction and purification methods from different humic acid-rich soils. J Appl Microbiol 102(1):265–273
Lieu PK, Homan H, Kurogi A, Kawagoshi Y, Fujii T, Furukawa K (2006) Characterization of sludge from single-stage nitrogen removal using anammox and partial nitrification (SNAP). Jpn J Water Treat Biol 42(2):53–64
Liu S, Gong Z, Yang F, Zhang H, Shi L, Furukawa K (2008) Combined process of urea nitrogen removal in anaerobic anammox co-culture reactor. Bioresour Technol 99(6):1722–1728
Mosquera-Corral A, González F, Campos JL, Méndez R (2005) Partial nitrification in a SHARON reactor in the presence of salinitys and organic carbon compounds. Process Biochem 40:3109–3118
Nakajima J, Sakka M, Kimura T, Furukawa K, Sakka K (2008) Enrichment of anammox bacteria from marine environment for the construction of a bioremediation reactor. Appl Microbiol Biotechnol 77:1159–1166
Neef A, Amann R, Schlesner H, Schleifer KH (1998) Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257–3266
Nielsen M, Bollmann A, Sliekers O, Jetten M, Schmid M, Strous M, Schmidt I, Larsen LH, Nielsen LP, Revsbech NP (2005) Kinetics, diffusional limitation and microscale distribution of chemistry and organisms in a CANON reactor. FEMS Microbiol Ecol 51:247–256
Pathak BK, Kazama F, Tanaka Y, Mori K, Sumino T (2007) Quantification of anammox populations enriched in an immobilized microbial consortium with low levels of ammonium nitrogen and at low temperature. Appl Microbiol Biotechnol 76:1173–1179
Peng Y, Zhu G (2006) Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl Microbiol Biotechnol 73:15–26
Pynaert K, Smets BF, Wyffels S, Beheydt D, Siciliano SD, Verstraete W (2003) Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Appl Environ Microbiol 69:3626–3635
Regan JM, Harrington GW, Noguera DR (2002) Ammonia-nitrite-oxidizing bacterial communities in a pilot-scale chloraminated drinking water distribution system. Appl Environ Microbiol 68:73–81
Rene ER, Kim SJ, Park HS (2008) Effect of COD/N ratio and salinity on the performance of sequencing batch reactors. Bioresour Technol 99:839–846
Schenk H, Hegemann W (1995) Nitrification inhibition by high salinity concentrations in the aerobic biological treatment of tannery wastewater. Wasser/Abwasser 136(9):465–470
Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M, Metzger JW, Scheleifer KH, Wagner M (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23:93–106
Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65(8):3690–3696
Semmens MJ, Porter PS (1979) Ammonium removal by ion exchange: using biologically restored regenerant. J Water Pollut Control Fed 51(12):2928–2940
Sliekers AO, Derwort N, Gomez JL, Strous M, Kuenen JG, Jetten MS (2002) Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res 36:2475–2482
Sliekers AO, Haaijer SCM, Stafsnes MH, Kuenen JG, Jetten MSM (2005) Competition and coexistence of aerobic ammonium and nitrite oxidizing bacteria at low oxygen concentrations. Appl Microbiol Biotechnol 68:808–817
Third KA, Sliekers AO, Kuenen JG, Jetten MSM (2001) The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: interaction and competition between three groups of bacteria. Syst Appl Microbiol 24:588–596
Toh SK, Ashbolt NJ (2002) Adaptation of anaerobic ammonium-oxidizing consortium to synthetic coke-ovens wastewater. Appl Microbiol Biotechnol 59:344–352
Uygur A (2006) Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem 41:61–66
van de Graaf AA, de Bruijn P, Robertson LA, Jetten MSM, Kuenen JG (1996) Autotrophic growth of anaerobic ammonium-oxidizing microorganisms in a fluidized bed reactor. Appl Environ Microbiol 142(8):2187–2196
Vredenbregt LHJ (1997) Fluid bed biological nitrification and denitrification in high salinity wastewater. Water Sci Technol 36:93–100
Wagner M, Loy A (2002) Bacterial community composition and function in sewage treatment system. Environ Biotechnol 13:218–227
Wagner M, Rath G, Koops HP, Flood J, Amann R (1995) In situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol 18:251–264
Waki M, Tokutomi T, Yokoyama H, Tanaka Y (2007) Nitrogen removal from animal wastetreatment water by anammox enrichment. Bioresour Technol 98:2775–2780
Windey K, Bo ID, Verstraete W (2005) Oxygen-limited autotrophic nitrification-denitrification (OLAND) in a rotating biological contactor treating high-salinity wastewater. Water Res 39:4512–4520
Zhang HM, Xiao JN, Cheng YJ, Liu LF, Zhang XW, Yang FL (2006) Comparison between a sequencing batch membrane bioreactor and a conventional membrane bioreactor. Process Biochem 41:87–95
Acknowledgments
We would like to thank Professor Kenji Furukawa for kindly supplying the anammox bacteria in Faculty of Engineering, Kumamoto University, Kumamoto, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Yang, F., Gong, Z. et al. Assessment of the positive effect of salinity on the nitrogen removal performance and microbial composition during the start-up of CANON process. Appl Microbiol Biotechnol 80, 339–348 (2008). https://doi.org/10.1007/s00253-008-1536-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1536-9