Abstract
Background
Assessment of fetal adipose tissue gives information about the future metabolic health of an individual, with evidence that the development of this tissue has regional heterogeneity.
Objective
To assess differences in the proton density fat fraction (PDFF) between fetal adipose tissue compartments in the third trimester using water-fat magnetic resonance imaging (MRI).
Materials and methods
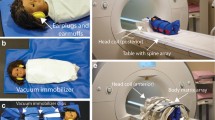
Water-fat MRI was performed in a 1.5-T scanner. Fetal adipose tissue was segmented into cheeks, thorax, abdomen, upper arms, forearms, thighs and lower legs. PDFF and R2* values were measured in each compartment.
Results
Twenty-eight women with singleton pregnancies were imaged between 28 and 38 weeks of gestation. At 30 weeks’ gestation (n=22), the PDFF was statistically different between the compartments (P<0.0001), with the highest PDFF in cheeks, followed by upper arms, thorax, thighs, forearms, lower legs and abdomen. There were no statistical differences in the rate of PDFF change with gestational age between the white adipose tissue compartments (P=0.97). Perirenal brown adipose tissue had a different PDFF and R2* compared to white adipose tissue, while the rate of R2* change did not significantly change with gestational age between white adipose tissue compartments (P=0.96).
Conclusion
Fetal adipose tissue accumulates lipids at a similar rate in all white adipose tissue compartments. PDFF variances between the compartments suggest that accumulation begins at different gestational ages, starting with cheeks, followed by extremities, trunk and abdomen. Additionally, MRI was able to detect differences in the PDFF between fetal brown adipose tissue and white adipose tissue.



Similar content being viewed by others
References
Wadhwa PD, Buss C, Entringer S, Swanson JM (2009) Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27:358–368
Ornoy A (2011) Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32:205–212
Ali AT, Hochfeld WE, Myburgh R, Pepper MS (2013) Adipocyte and adipogenesis. Eur J Cell Biol 92:229–236
Reeder SB, Hu HH, Sirlin CB (2012) Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging 36:1011–1014
Giza SA, Olmstead C, McCooeye DA et al (2018) Measuring fetal adipose tissue using 3D water-fat magnetic resonance imaging: a feasibility study. J Matern Fetal Med 33:831–837
Hu HH, Yin L, Aggabao PC et al (2013) Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI. J Magn Reson Imaging 38:885–896
Anblagan D, Deshpande R, Jones NW et al (2013) Measurement of fetal fat in utero in normal and diabetic pregnancies using magnetic resonance imaging. Ultrasound Obstet Gynecol 42:335–340
Deans HE, Smith FW, Lloyd DJ et al (1989) Fetal fat measurement by magnetic resonance imaging. Br J Radiol 62:603–607
Jovanovic-Peterson L, Crues J, Durak E, Peterson CM (1993) Magnetic resonance imaging in pregnancies complicated by gestational diabetes predicts infant birthweight ratio and neonatal morbidity. Am J Perinatol 10:432–437
Berger-Kulemann V, Brugger PC, Reisegger M et al (2012) Quantification of the subcutaneous fat layer with MRI in fetuses of healthy mothers with no underlying metabolic disease vs. fetuses of diabetic and obese mothers. J Perinat Med 40:179–184
Larciprete G, Di Pierro G, Barbati G et al (2008) Could birthweight prediction models be improved by adding fetal subcutaneous tissue thickness? J Obstet Gynaecol Res 34:18–26
Hu HH, Perkins TG, Chia JM, Gilsanz V (2013) Characterization of human brown adipose tissue by chemical-shift water-fat MRI. AJR Am J Roentgenol 200:177–183
Yu H, McKenzie CA, Shimakawa A et al (2007) Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 26:1153–1161
Reeder SB, Pineda AR, Wen Z et al (2005) Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med 54:636–644
Reeder SB, McKenzie CA, Pineda AR et al (2007) Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging 25:644–652
Yu H, Shimakawa A, Hines CD et al (2011) Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med 66:199–206
Yu H, Shimakawa A, McKenzie CA et al (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60:1122–1134
3D Slicer homepage. www.slicer.org. Accessed 6 Dec 2016
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341
Kikinis R, Pieper S, Vosburgh K (2014) 3D slicer: a platform for subject-specific image analysis, visualization, and clinical support. In: Jolesz FA (ed) Intraoperative imaging image-guided therapy. Springer, New York
Roberts NT, Hernando D, Holmes JH et al (2018) Noise properties of proton density fat fraction estimated using chemical shift-encoded MRI. Magn Reson Med 80:685–695
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Poissonnet CM, Burdi AR, Garn SM (1984) The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev 10:1–11
Blondiaux E, Chougar L, Gelot A et al (2018) Developmental patterns of fetal fat and corresponding signal on T1-weighted magnetic resonance imaging. Pediatr Radiol 48:317–324
Hu HH, Tovar JP, Pavlova Z et al (2012) Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging 35:938–942
Merklin RJ (1974) Growth and distribution of human fetal brown fat. Anat Rec 178:637–645
Modi N, Murgasova D, Ruager-Martin R et al (2011) The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res 70:287–291
Sewell MF, Huston-Presley L, Super DM, Catalano P (2006) Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 195:1100–1103
Hull HR, Dinger MK, Knehans AW et al (2008) Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol 198:416 e411-416
Benkert T, Feng L, Sodickson DK et al (2016) Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med 78:565–576
Acknowledgments
We thank Jennifer Ryder and Laura McMurphy for their help with recruitment, scheduling of MRIs and data collection. We also thank Samantha Bedell for her help in preparing this manuscript. Funding came from Western University’s Strategic Support for Canadian Institutes of Health Research (CIHR) Success Program, Children’s Health Research Institute Translational Research Grant Fund, CIHR/Institute of Human Development, Child and Youth Health/Society of Obstetricians and Gynaecologists of Canada Maternal Fetal Medicine Team grant, University of Western Ontario Internal Schulich School–internal funding–dean’s support, Children’s Health Research Institute (CHRI)/Children’s Health Foundation, Women’s Development Council (London Health Sciences Centre), and the Department of Obstetrics and Gynaecology of Western University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giza, S.A., Koreman, T.L., Sethi, S. et al. Water-fat magnetic resonance imaging of adipose tissue compartments in the normal third trimester fetus. Pediatr Radiol 51, 1214–1222 (2021). https://doi.org/10.1007/s00247-020-04955-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04955-z