Abstract
Assessing heart failure progression in patients with Duchenne Muscular Dystrophy (DMD) is challenging given the multi-system nature of disease. Herein we describe the first case use of an implantable pulmonary artery pressure monitor and describe the potential clinical utility of this approach in patients with DMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case Report
The patient is a 23-year-old male with DMD who had been managed on chronic oral steroids since age 3. Of note, he lived remote from any major medical center, and owing to logistic difficulties, was only able to attend clinic periodically. Due to significant progressive weakness, he had been managed at home with sip ventilator during the day and bi-level ventilation at night. His most recent forced vital capacity from 7 years prior was 0.9 L (22% predicted). At presentation, he was on carvedilol 9.375 mg twice daily, lisinopril 2.5 mg daily, and spironolactone 25 mg daily. He had previously tolerated doses as high as carvedilol 12.5 mg twice daily and lisinopril 10 mg daily, but those doses had been sequentially decreased due to symptomatic hypotension since an admission for pneumonia 5 years prior to his current presentation.
On presentation to clinic, he complained of acute on chronic (> 3 years) fatigue and lower extremity pitting edema. Over the prior 6 months he described progressive cough and orthopnea. He also noted swelling and intermittent orthopnea since the previously noted admission for pneumonia.
His clinical exam was remarkable for a pulse of 84 beats/min, blood pressure 124/86 mmHg, oxygen saturations of 97% in room air, a weight of 103.9 kg and a BMI of 41 kg/m2. Jugular venous distention and hepatomegaly could not be assessed due to his body habitus/body position. Mild pitting edema was noted to the calf bilaterally, as well as non-pitting edema of the arms to the level of the bicep. There was no peripheral hyperpigmentation or ulceration noted.
His nt-BNP was 150 pg/mL, similar to the year prior (128 pg/mL) and slightly elevated from a measurement 5 years prior (109 pg/mL). Additional laboratory evaluation including hepatic and renal function were unchanged. Cystatin C GFR was stable over the previous 5 years (85–92 mL/min/1.73 m2) and the total bilirubin was normal. His cardiac MRI demonstrated a LVEF of 39% and RVEF of 51% in the absence of ventricular dilation (left ventricular end diastolic volume 68 mL/m2, right ventricular end diastolic volume 55 mL/m2). These findings were stable when compared with serial cardiac MRIs over the previous 3 years.
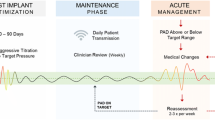
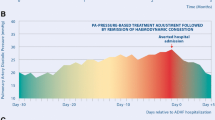
He was initially managed with changes in salt and fluid intake as well as clinically driven diuretic management. However, due to persistence of symptoms and the challenges in differentiating heart failure and respiratory/swallow symptoms, the patient underwent right heart catheterization under minimal sedation. Hemodynamics revealed a low thermodilution cardiac index of 2.34 L/min/m2, and elevated right and left ventricular filling pressures [mean right atrial pressure 15 mmHg, mean pulmonary artery wedge pressure 27 mmHg, and a normal pulmonary vascular resistance (2.5 Wu m2)]. During the cardiac catheterization, and after a pulmonary angiogram demonstrated an appropriately sized left lower pulmonary artery (greater than 7 mm in diameter), a wireless pulmonary artery pressure device (CardioMEMS, Abbott Laboratories, Atlanta, GA) was implanted and calibrated successfully (Fig. 1).
The patient had an uneventful recovery from the catheterization and was discharged home on increased diuretic therapy. During the 24 months of follow-up, the patient has been able to transmit pulmonary artery recordings at regular intervals (ultimately spaced out to every 2–4 weeks once he was stable). Briefly, changes in this patient’s symptoms, such as worsening cough or dyspnea were compared to pulmonary artery pressure recordings, and in cases of increased pressure and symptomatic worsening, diuretic dosing was increased. Titrations such as these were managed remotely without requiring patient travel. The symptoms of cough and dyspnea have now completely resolved, and he has not required any further admissions.
Discussion
This case represents the first known implantation of a wireless pulmonary artery pressure monitor in a patient with DMD. The use of this technology has not previously been reported in this patient population, and this case report demonstrates the potential utility of remote invasive monitoring given the challenges in assessing heart failure symptoms and medication management in non-ambulatory DMD patients with advanced multi-system disease.
In DMD, symptom assessment and medication titration may be challenging due to the nature of this multi-system disease. Furthermore, typical biomarkers of disease progression in DMD may be hard to interpret, and invasive hemodynamic assessment may be required to discern the contribution of heart failure to disease symptoms.
Improvement in respiratory and neuromuscular care have extended survival in DMD by delaying the onset of respiratory failure [1]. These improvements have unmasked the progression of cardiomyopathy which typically begins in the teenage years and progresses with age [2]. By the time cardiomyopathy progresses, patients often are non-ambulatory and have appreciable respiratory weakness. The challenges in documenting disease progression and attributing symptom progression to cardiac disease are further complicated by the limitations of physical examination, and laboratory and radiographic testing in patients with advanced disease. Furthermore, obesity commonly limits clinical assessment of jugular venous distention, while lung volume loss and scoliosis/patient positioning can make assessment of hepatomegaly a challenge. Lower extremity edema is common in DMD, especially in patients with long standing loss of ambulation and minimal residual lower extremity strength [3]. Additionally, there are no comprehensive studies describing the progression of laboratory biomarkers of disease progression such as natriuretic peptides. Existing case series suggest modified “normal values” may be needed in DMD, as appreciable rises may be a late finding [4]. Similarly, in our case, persistently low nt-BNP values (100–150 pg/mL) and “stably” depressed left ventricular systolic dysfunction (LVEF 40% ± 2%) had provided false reassurance that his symptoms were driven by respiratory progression rather than heart. In his case, cardiac catheterization was immensely useful in understanding the cardiac contribution to his overall disease process.
The implantation of the remote monitoring device allowed for successful outpatient management. Considering his course since implantation, with diuretic adjustment, and with no further admissions for pneumonia or respiratory disease (with no changes in pulmonary management), we believe this patient had been subject to undiagnosed pulmonary venous congestion secondary to left atrial hypertension that contributed to his respiratory symptoms and pulmonary admissions for many years. His case is consistent with published data indicating that remote patient monitoring using pulmonary artery pressure monitoring is associated with lower heart failure hospitalizations and heart failure related costs in non-DMD patients [5,6,7].
This case, as well as the evolving clinical experience suggests that centers should consider cardiac catheterization with potential use of implantable pressure monitors where applicable to help discern the potential cardiac contribution to respiratory symptoms. We would make the further argument that this should be done earlier in the course of the disease when the risk of the initial catheterization would be substantially lower. It is also worth noting, that contractures and/or appreciable scoliosis would be potential concerns in this population. Fortunately, this patient had no contractures impacting the ability to access the vasculature, and had his scoliosis addressed previously.
Finally, while there is ongoing debate regarding the impact of remote patient monitoring in specific populations, this case demonstrates its potential use in a rare disease cohort that may have limited alternative options. This approach is also finding potential application in children with congenital heart disease and heart failure [8].
Conclusion
This patient’s case suggests implantable pulmonary artery pressure guided management can assist clinical management for DMD patients. The potential utility of this approach is especially important since many adults with DMD have limited local access to adult care providers with experience in DMD related heart failure, and long-distance management is thus attainable. Further study of this innovative approach is warranted, and collective experience to understand the overall impact is vitally important.
Abbreviations
- DMD:
-
Duchenne muscular dystrophy
- GFR:
-
Glomerular filtration rate
- kg:
-
Kilogram
- LVEF:
-
Left ventricular ejection fraction
- L:
-
Liter
- m:
-
Meter
- mg:
-
Milligram
- min:
-
Minute
- mL:
-
Milliliter
- mm:
-
Millimeter
- mmHg:
-
Millimeters of mercury
- MRI:
-
Magnetic resonance imaging
- nt-BNP:
-
Aminoterminal B-type natriuretic peptide
- PA:
-
Pulmonary artery
- pg:
-
Picogram
- RVEF:
-
Right ventricular ejection fraction
- Wu:
-
Wood unit
References
Broomfield J, Hill M, Guglieri M, Crowther M, Abrams K (2021) Life expectancy in Duchenne muscular dystrophy: reproduced individual patient data meta-analysis. Neurology 97(23):e2304–e2314. https://doi.org/10.1212/WNL.0000000000012910. (Epub 15 Oct 2021)
Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA et al (2015) Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. J Am Heart Assoc. https://doi.org/10.1161/JAHA.114.001338. (Epub 31 March 2015)
Freytag JM, Ryan TD, Bange JE, Bonarrigo KC, Chouteau WA, Wittekind SG et al (2022) Obesity and loss of ambulation are associated with lower extremity oedema in Duchenne muscular dystrophy. Cardiol Young. https://doi.org/10.1017/S1047951122001342. (Epub 14 May 2022)
Cha JJ, Kim IS, Kim JY, Choi EY, Min PK, Yoon YW et al (2022) The association between cardiac involvement and long-term clinical outcomes in patients with Duchenne muscular dystrophy. ESC Heart Fail. https://doi.org/10.1002/ehf2.13970. (Epub 18 May 2022)
Dauw J, Sokolski M, Middleton JT, Nijst P, Dupont M, Forouzan O et al (2022) Ambulatory haemodynamic-guided management reduces heart failure hospitalizations in a multicentre European heart failure cohort. ESC Heart Fail 9(6):3858–3867. https://doi.org/10.1002/ehf2.14056. (Epub 3 Aug 2022)
Heywood JT, Zalawadiya S, Bourge RC, Costanzo MR, Desai AS, Rathman LD et al (2023) Sustained reduction in pulmonary artery pressures and hospitalizations during 2 years of ambulatory monitoring. J Card Fail 29(1):56–66. https://doi.org/10.1016/j.cardfail.2022.10.422. (Epub 5 Nov 2022)
Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD et al (2020) Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail 13(8):e006863. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006863. (Epub 8 Aug 2020)
Bhat DP, Graziano JN, Garn BJ, Franklin WJ (2023) Safety and utility of CardioMEMS device for remote pulmonary artery monitoring in paediatric Fontan patients: a case series. Eur Heart J Case Rep 7(9):ytad422. https://doi.org/10.1093/ehjcr/ytad422
Funding
No funding was secured for this study.
Author information
Authors and Affiliations
Contributions
T.S. and C.V. wrote the main manuscript text. R.H. prepared figure 1. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Lorts is a Consultant for Abbott Laboratories and has also received a research grant from Abbott Laboratories. The other authors have no relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sisson, T.M., Sublett-Smith, J., Dupont, E. et al. Wireless Pulmonary Artery Pressure Monitor Implantation in a Patient with Duchenne Muscular Dystrophy. Pediatr Cardiol 45, 1151–1153 (2024). https://doi.org/10.1007/s00246-024-03459-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-024-03459-z