Abstract
In this updated meta-analysis, we assessed the cardioprotective effect of remote ischemic preconditioning (RIPC) in pediatric patients undergoing congenital heart surgery. A total of 9 randomized controlled trials (RCTs) involving 793 pediatric patients under 18 years old were identified. RIPC obviously reduced the release of troponin I at 6 h after surgery [standard mean difference (SMD) −0.59, 95% confidence interval (CI) −1.14 to −0.04; p = 0.03], mitigated the inotropic scores within 4–6 h (SMD −0.43, 95% CI −0.72 to −0.14; p = 0.004) and within 12 h (SMD −0.26, 95% CI −0.50 to −0.02; p = 0.03) and shortened the ventilator support time (SMD −0.28, 95% CI −0.49 to −0.07; p = 0.01) as well as the duration of intensive care unit (ICU) stay (SMD −0.21, 95% CI −0.35 to −0.06; p = 0.004). Our meta-analysis determined that RIPC had cardioprotective effects in the early postoperative phase. Additional RCTs focused on the cardiac benefits from RIPC in pediatric patients are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart disease (CHD) occurs in 4–50 cases per 1000 live births, and it is the one of the most common causes of infant death [1]. Children appear to be more vulnerable to cardiac dysfunction when they undergo cardiac surgical procedures [2, 3], and abnormally elevated increases of myocardial enzymes are closely related to worse outcome in both the early and later postoperative phases [4, 5]. Moreover, complications following myocardial injury are also associated with prolonged ventilator support time, prolonged intensive care unit (ICU) and hospital stays and even increased mortality.
There are various approaches to preventing myocardial injury following cardiac operations, including minimizing the duration of aortic cross-clamping, the modulation of temperature (cold, tepid or warm), intermittent or continuous infusion, the selection of crystalloid or blood cardioplegia and pharmacological pretreatment [6]. Unfortunately, there are few absolutely effective methods for the mitigation of myocardial damage.
Remote ischemic preconditioning (RIPC) is originated from local ischemic preconditioning and is a noninvasive and nonpharmacologic method that has been reported to be promising and feasible for cardioprotection in animal experiments since the mid-1980s. RIPC was first employed in patients in 2006, which led to many studies regarding its use. RIPC might prevent the heart from injury via activating neuronal or humoral pathways or systemic responses that release anti-inflammatory cytokines and prosurvival signals [7]; thus, RIPC might serve as a noninvasive method for the prevention of myocardial damage in patients undergoing cardiac surgery.
To date, several clinical trials seeking to determine the role played by RIPC in pediatric postoperative cardioprotection have been published. However, the published data lack strong evidence and a uniform conclusion has not been reached; thus, the result remains controversial. Therefore, our primary aim was to systematically review and demonstrate the recent evidence of postoperative cardioprotective effects from RIPC in pediatric patients who have undergone congenital heart surgery [the index included the release of troponin I (cTnI) and inotropic scores]. As a secondary aim, we investigated the ventilation support time, duration of the ICU stays and length of hospital stays with the intervention of RIPC in these clinical trials.
Methods
Study Selection and Outcome Measures
Eligible studies were required to possess the following characteristics: (1) all patients were children (under 18 years old); (2) they underwent surgical procedures to address congenital heart defects, such as ventricular septal defects (VSD), atrioventricular septal defects (ASD), tetralogy of Fallot (TOF), aortic regurgitation (by valve repair) and transposition of the great arteries; (3) RIPC was used as an intervention in the upper or lower limbs; and (4) the studies were RCTs for the detection of myocardial injury following congenital cardiac surgery.
The primary endpoints were the release of cTnI and the inotropic scores. The secondary endpoints were the ventilation support time and the duration of ICU and hospital stays.
Search Strategy
The PubMed, Embase and Cochrane databases were screened until April 16, 2017. The search terms were as follows: ((((((((((cardiovascular surgical procedures) OR cardiac surgical procedures) OR cardiac surgery) OR heart surgery) OR ventricular septal defects) OR atrial septal defects) OR tetralogy of Fallot) OR cardiopulmonary bypass)) AND ((((ischemic preconditioning) OR myocardial ischemic preconditioning) OR remote ischemic preconditioning) OR limb ischemic preconditioning)) AND (((((((((child) OR children) OR infant) OR infants) OR newborn) OR newborns) OR neonate) OR neonates) OR pediatrics). The references of recent review articles and the included trials were searched for additional studies. The abstracts and titles were repeatedly checked by two of the authors (W.T. and C.Z.) to guarantee their adherence to the inclusion criteria. The full texts were carefully reviewed if the articles possessed vague titles or abstracts.
Study Quality Assessment
The quality of the included studies was measured using the Cochrane risk of bias criteria. Two investigators (W.T. and C.Z.) independently evaluated the quality of these studies. The criteria included random sequence generation and allocation concealment (selection bias); blinding of personnel and participation (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attribution bias); selective reporting (reporting bias); and other bias. Then, the risks were graded as low risk, high risk or unclear risk.
Data Extraction
Two reviewers (W.T. and C.Z.) extracted data from the eligible studies utilizing uniform standard criteria and by reaching a consensus. An arbitrator (Q.M.) resolved any disputes between the reviewers. Each of the reviewers independently evaluated every trial and extracted data on the basic characteristics of the patients (e.g., age, sex and country), type of operation, type of anesthesia, anesthetic regimen, RIPC protocol, release of cTnI, inotropic scores, mechanical ventilation time and duration of the ICU and hospital stays after surgery. Any missing data and supplemental information required from the original studies were requested from the author via email. In cases where there were no responses, we used arithmetic or graphic approximation methods to determine missing values, and medians and interquartile ranges were transformed into the mean and SD.
Data Analysis
The data from the studies were analyzed using Review Manager (RevMan) version 5.3. We evaluated the collected data using the standard mean difference (SMD) with 95% confidence intervals (CIs) in the inverse variance method with a random-effects analysis model. Statistical heterogeneity was assessed using the χ2 test and Ι2 statistics. Values of p < 0.10 for the test of heterogeneity were considered to be statistically significant. Moreover, the heterogeneity was considered mild if Ι2 ≥ 25%; moderate if Ι2 ≥ 50%; and high if Ι2 ≥ 75%. If heterogeneity was detected, we performed a sensitivity analysis by excluding one study at a time from the pooled analysis.
The protocol for our meta-analysis was registered on PROSPERO (ID: CRD42017070210) and can be accessed at http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42017070210.
Results
Study Selection and Identification
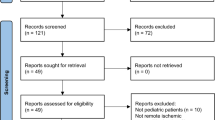
A total of 157 publications were identified from the initial database search [PubMed (n = 82), Embase (n = 73) and Cochrane (n = 2)]. Among those, 32 articles were first excluded due to duplicate studies, and then, 98 articles were excluded after screening the titles or abstracts. Among the remaining 27 articles, 4 articles were conference papers, 3 were letters to the editor, 5 were irrelevant papers, 2 involved irrelevant interventions, 1 was a meta-analysis and 1 was an animal experiment; these were excluded after screening the full text. The remaining 11 papers were eligible randomized controlled trials (RCTs). However, data from one study were presented as a mean difference with the 95% CI instead of the mean with SD [8] and data from another study were presented by a box plot [9]; as a result, we could not collect data from these two papers, and thus, the papers were excluded. Finally, nine eligible RCTs were selected and included in the meta-analysis. The process of identifying eligible RCTs is presented in Fig. 1.
Characteristics of the Eligible Studies
Table 1 displays the basic characteristics of the eligible studies. These studies were published from 2006 to 2017 and included 793 children in 5 countries. The sample sizes ranged from 37 to 299. The intervention of RIPC in all studies included three cycles or four cycles of ischemia–reperfusion for 5 min using a sphygmomanometer cuff inflated to over 200 or 15–40 mmHg above systolic pressure on the upper or lower limbs. The RIPC method varied among these trials: six trials [10,11,12,13,14,15] applied four cycles of intervention, three trials [16,17,18] applied three cycles, eight trials [10,11,12,13,14,15,16, 18] applied RIPC on the lower limbs and one trial [17] applied RIPC on the upper limbs. Among the 9 included studies, 4 studies [10,11,12, 17] evaluated the concentration of cTnI at 6 h, 5 studies measured the release of cTnI at 12 [10,11,12,13, 16] and 24 h [10,11,12,13, 17] after surgery; 5 articles [10, 11, 13, 17, 18] reported the inotropic scores at 4–6 and 12 h; 6 articles [10,11,12,13, 17, 18] reported the inotropic scores at 24 h; 6 articles [11,12,13, 16,17,18] described the mechanical ventilation time; 8 articles [11,12,13,14,15,16,17,18] reported the duration of the ICU stay; and 6 articles [12,13,14,15,16, 18] reported the duration of the hospital stay.
Quality Assessment
According to the criteria of the Cochrane handbook of bias assessment, the final risk of bias summary and risk of bias graph are shown in Fig. 2. The selection bias was determined to be low risk, as the percentage of random sequence generation was 56% and the allocation concealment was 67%. Performance bias evaluated by the blinding of personnel and participants was identified as low risk at 89%. However, the detection and reporting biases were unclear in over half of the included studies due to limited information. Moreover, the unclear risk of attrition bias remained high at 56% due to insufficient information of the reported outcome.
Postoperative Cardiac Troponin I
A total of 6 RCTs [10,11,12,13, 16, 17] involving 277 pediatric patients were used to assess the effect of RIPC on the postoperative release of cTnI. Overall, RIPC decreased the concentrations of cTnI at 6 h after congenital heart surgery (SMD −0.59, 95% CI −1.14 to −0.04; heterogeneity χ2 = 15.43, Ι2 = 74%, PH = 0.004; p = 0.03, Fig. 3a). However, RIPC could not decrease the release of cTnI at 12 h (SMD −0.61, 95% CI −1.28 to 0.05; heterogeneity χ2 = 15.17, Ι2 = 80%, PH = 0.002; p = 0.07, Fig. 3b) and 24 h (SMD −0.18, 95% CI −0.72 to 0.37; heterogeneity χ2 = 17.10, Ι2 = 77%, PH = 0.002; p = 0.53, Fig. 3c). Sensitivity analysis by omitting one article at a time showed that the outcomes became nonsignificant at 6 h when the study by Cheung et al. was removed.
Postoperative Inotropic Scores
A total of 5 studies [10, 11, 13, 17, 18] involving 304 pediatric patients reported the inotrope scores at 4–6 h and 12 h, and 6 studies [10,11,12,13, 17, 18] involving 349 patients reported the inotrope scores at 24 h. The pooled results showed that RIPC decreased postoperative inotropic scores at 4–6 (SMD −0.43, 95% CI −0.72 to −0.14; heterogeneity χ2 = 6.06, Ι2 = 34%, PH = 0.19; p = 0.004, Fig. 4a) and 12 h (SMD −0.26, 95% CI −0.50 to −0.02; heterogeneity χ2 = 4.35, Ι2 = 8%, PH = 0.36; p = 0.03, Fig. 4b), but the protective effect of RIPC was not observed at 24 h (SMD −0.02, 95% CI −0.25 to 0.22; heterogeneity χ2 = 6.05, Ι2 = 17%, PH = 0.30; p = 0.89, Fig. 4c).
Ventilator Support Time
A total of 6 RCTs [11,12,13, 16,17,18] involving 352 pediatric patients reported the ventilation support time after cardiac surgery. The RIPC intervention was associated with a shorter ventilator support time (SMD −0.28, 95% CI −0.49 to −0.07; heterogeneity χ2 = 3.76, Ι2 = 0%, PH = 0.59; p = 0.01, Fig. 5a).
Duration of the ICU Stay
The outcomes of 8 studies [11,12,13,14,15,16,17,18] involving 756 pediatric patients showed that RIPC reduced the duration of the ICU stay after cardiac surgery (SMD −0.21, 95% CI −0.35 to −0.06; heterogeneity χ2 = 5.08, Ι2 = 0%, PH = 0.65; p = 0.004, Fig. 5b).
Hospital Stay
The pooled results of 6 RCTs [12,13,14,15,16, 18] with 661 patients did not show a significant decrease in the duration of hospital stay when the patients received RIPC intervention before cardiac surgery (SMD −0.24, 95% CI −0.49 to 0.01; heterogeneity χ2 = 10.99, Ι2 = 55%, PH = 0.05; p = 0.06, Fig. 5c). However, when the study by McCrindle et al. was omitted, RIPC was shown to reduce the duration of hospital stay (SMD −0.32, 95% CI −0.53 to −0.11; heterogeneity χ2 = 3.92, Ι2 = 0%, PH = 0.42; p = 0.003).
Discussion
We selected relevant RCTs to assess the cardioprotective effect of RIPC in pediatric patients undergoing congenital heart surgical procedures. Compared with the control group, our meta-analysis suggested that RIPC had a cardioprotective effect in children during the early postoperative phase. cTnI release at 6 h and the inotropic scores at 4–6 h strongly suggested the beneficial cardioprotective effects of RIPC. In addition, RIPC also deceased the inotrope scores at 12 h, the ventilator support time and the duration of the ICU stay, but it did not reduce the duration of the hospital stay.
Troponin I (cTnI) is the first choice and most reported parameter for the detection of myocardial damage due to its specificity and sensitivity [19]. In our meta-analysis, RIPC mitigated the release of cTnI at 6 h; however, it did not reduce the release of cTnI at 12 and 24 h. A possible reason was that the increase of cTnI concentration was fastest within 6 h, and the peak value appeared at 12–24 h after surgery [20]. Due to the presence of high heterogeneity, we removed one article (Cheung et al.) from the meta-analysis, and the heterogeneity decreased to 0. Although the outcome might be more reliable and stable if the RCT by Cheung et al. was excluded, we should be cautious of the deletion, as the RCT complied with our inclusion criteria.
The postoperative cTnI is a beneficial tool for reassessment after cardiac surgery. It is noninvasive, easily obtained and allows for prediction of patients at risk for death and major adverse cardiac events (MACEs) within a few months [21]. However, the source of troponin during cardiac surgery is still a controversy. Although some argue that troponin is released from damaged cardiac myocytes, it has also been indicated that increased permeability of the cell causes leakage of troponin from the cytosol [22]. Regardless of the source, previous studies have determined that troponin release following cardiac surgery has also been independently associated with short-term and long-term outcomes postoperatively, such as morbidity and mortality [23]. In addition, the postoperative troponin levels have been used in a majority of RIPC meta-analyses in cardiac surgery as the primary outcomes [24, 25]. From the clinical perspective, equating the increased troponin with myocardial injury still makes a good deal of clinical sense.
The inotropic scores were also used as an additional tool to measure illness severity during the postoperative period [26]. The inotropic score = (dopamine + dobutamine) × 1 + milrinone × 10 + epinephrine × 100, and a higher inotropic score reflects a greater degree of myocardial dysfunction. Reduced inotropic scores at 4–6 h were in accordance with the time window of decreased cTnI. In addition, the data also showed that RIPC decreased inotropic scores at 12 h and shortened the duration of mechanical ventilation and the ICU stay, which are indirect indices of postoperative complications [27] in our pooled analysis. All the summarized outcomes suggested that RIPC, which may cause the transfer of protective signals to remote organs via neuronal and humoral communication [28], exhibited an overall positive effect, especially during the early phase after cardiac surgery.
Regarding the duration of hospital stay, McCrindle et al. analyzed their trial data by subgroup analyses and the stratification of confounding factors, including age, sex, type of surgery, Aristotle level, duration of cardiopulmonary bypass or aortic cross-clamping and the perioperative use of propofol. RIPC was not found to have any statistically significant effect in any of these subgroup analyses. Due to its large sample size of 299 pediatric patients, which accounted for 25.9% of the final outcome in our analysis, the stability of the pooled effect was influenced by the inclusion of this trial. Despite the moderate heterogeneity, this trial could not be excluded because the summarized data on the duration of the hospital stay may be an authentic outcome.
Following its use in adult patients, RIPC was adapted for application to children under 18 years old who were undergoing congenital heart surgery. Recently, a meta-analysis that used the release of troponin I (cTnI) as the primary endpoint evaluated the cardioprotective effect of RIPC in children; unfortunately, the outcomes were controversial [25]. Moreover, with the new RCTs that were published this year, an updated meta-analysis was needed. Our meta-analysis extended the previous pooled analysis in several ways. First, by adding 2 new papers including 157 pediatric patients, the statistical power was increased for the determination of myocardial protective results. Second, other representative clinical endpoints, such as the inotropic scores, mechanical ventilator support time and duration of the ICU and hospital stay, were collected for a more comprehensive assessment.
Nevertheless, there were several limitations in our meta-analysis. First, troponin release can be affected by different type of congenital heart surgery. Higher troponin levels are seen post-operatively in surgeries that require direct incisions on the myocardium (such as VSD and ASD) and are lower in surgeries that mostly work on the major vascular structures (such as extracardiac Fontan operation and the Glenn procedure). Due to lack of adequate information, we were not sure these surgeries were balanced in the two groups. Second, we could not perform a subgroup analysis to exclude confounders due to a limited number of studies. Previous meta-analyses have also failed to determine the cardioprotective effect of RIPC due to the presence of numerous confounders in the included studies, including age, type of congenital heart surgery and anesthesia regimen (volatile anesthesia and propofol), which could mask the effect of RIPC or interfere with RIPC [28]. Third, due to the lack of replies from several authors, we employed the graphic approximation method to acquire data, and although two data collectors (X.L. and Y.C.) performed the data approximation collection, there might be some errors. Finally, we omitted one study at a time from our meta-analysis for the sensitivity and heterogeneity analyses. Although excluding one study could reduce the heterogeneity, we still should be cautious when drawing conclusions.
Several meta-analyses on adult patients have reported that RIPC could reduce the release of cTnI after cardiovascular surgery [29, 30] and even reduce the risk for cardiovascular events and acute kidney injury [31,32,33]. Although the effect of RIPC is still controversial, the latest meta-analysis revealed that after excluding interference from the anesthesia regimen (propofol), RIPC was observed to be beneficial for adults undergoing cardiac surgery [34]. Beta-blockers and volatile anesthetics may also attenuate the effect of RIPC [35]. Moreover, RIPC seemed to be more effective when the exposure time was longer and the volume of tissue undergoing RIPC was greater [36]. If RIPC is combined with remote ischemic postconditioning, the protective effect may be more obvious [37,38,39,40]. Currently, there are registered clinical trials investigating the protective effect of RIPC on other organs, including neuroprotection and kidney protection. We anticipate that the clinical outcomes of these new trials may extend the benefits of RIPC.
In summary, the limited evidence in our meta-analysis suggests that RIPC might offer cardioprotection by reducing the release of cTnI and by reducing the inotropic scores during the early postoperative phase. In addition, RIPC benefited children undergoing cardiac surgery by shortening the ventilator support time and the duration of the ICU stay. Additional large-scale, high-quality RCTs are required in the near future to assess the effect of RIPC on the early and late postoperative phases in children.
References
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8:50–60
Hammer S, Loeff M, Reichenspurner H, Daebritz S, Tiete A, Kozlik-Feldmann R, Reichart B, Netz H (2001) Effect of cardiopulmonary bypass on myocardial function, damage and inflammation after cardiac surgery in newborns and children. Thorac Cardiovasc Surg 49:349–354
Hasegawa T, Yamaguchi M, Yoshimura N, Okita Y (2005) The dependence of myocardial damage on age and ischemic time in pediatric cardiac surgery. J Thorac Cardiovasc Surg 129:192–198
Moon MH, Song H, Wang YP, Jo KH, Kim CK, Cho KD (2014) Changes of cardiac troponin I and operative mortality of coronary artery bypass. Asian Cardiovasc Thorac Ann 22:40–45
Lasocki S, Provenchere S, Benessiano J, Vicaut E, Lecharny JB, Desmonts JM, Dehoux M, Philip I (2002) Cardiac troponin I is an independent predictor of in-hospital death after adult cardiac surgery. Anesthesiology 97:405–411
Yamamoto H, Yamamoto F (2013) Myocardial protection in cardiac surgery: a historical review from the beginning to the current topics. Gen Thorac Cardiovasc Surg 61:485–496
Liu Z, Gong R (2015) Remote ischemic preconditioning for kidney protection: GSK3beta-centric insights into the mechanism of action. Am J Kidney Dis 66:846–856
Jones BO, Pepe S, Sheeran FL, Donath S, Hardy P, Shekerdemian L, Penny DJ, McKenzie I, Horton S, Brizard CP, d’Udekem Y, Konstantinov IE, Cheung MM (2013) Remote ischemic preconditioning in cyanosed neonates undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg 146:1334–1340
Pavione MA, Carmona F, de Castro M, Carlotti AP (2012) Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg 144:178–183
Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282
Lee JH, Park YH, Byon HJ, Kim HS, Kim CS, Kim JT (2012) Effect of remote ischaemic preconditioning on ischaemic-reperfusion injury in pulmonary hypertensive infants receiving ventricular septal defect repair. Br J Anaesth 108:223–228
Guerra GG, Joffe AR, Seal R, Phillipos E, Wong M, Moez EK, Dinu IA, Duff JP, Ross D, Rebeyka I, Robertson CM (2017) Pilot randomized controlled trial on early and late remote ischemic preconditioning prior to complex cardiac surgery in young infants. Paediatr Anaesth 27:433–441
Pepe S, Liaw NY, Hepponstall M, Sheeran FL, Yong MS, d’Udekem Y, Cheung MM, Konstantinov IE (2013) Effect of remote ischemic preconditioning on phosphorylated protein signaling in children undergoing tetralogy of Fallot repair: a randomized controlled trial. J Am Heart Assoc 2:e000095
Pedersen KR, Ravn HB, Povlsen JV, Schmidt MR, Erlandsen EJ, Hjortdal VE (2012) Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: a randomized single-center study. J Thorac Cardiovasc Surg 143:576–583
McCrindle BW, Clarizia NA, Khaikin S, Holtby HM, Manlhiot C, Schwartz SM, Caldarone CA, Coles JG, Van Arsdell GS, Scherer SW, Redington AN (2014) Remote ischemic preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass: a single-center double-blinded randomized trial. J Am Heart Assoc 3:e000964
Luo W, Zhu M, Huang R, Zhang Y (2011) A comparison of cardiac post-conditioning and remote pre-conditioning in paediatric cardiac surgery. Cardiol Young 21:266–270
Wenwu Z, Debing Z, Renwei C, Jian L, Guangxian Y, Pingbo L, Xinmin Z (2010) Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol 31:22–29
Wu Q, Wang T, Chen S, Zhou Q, Li H, Hu N, Feng Y, Dong N, Yao S, Xia Z (2017) Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of Fallot repair surgery: a randomized controlled trial. Eur Heart J 21(10):2503–2513
Paparella D, Guida P, Caparrotti S, Fanelli V, Martinelli G, Mazzei V, Zaccaria S, Bisceglia L, Scrascia G (2014) Myocardial damage influences short- and mid-term survival after valve surgery: a prospective multicenter study. J Thorac Cardiovasc Surg 148:2373–2379
Di Stefano S, Casquero E, Bustamante R, Gualis J, Carrascal Y, Bustamante J, Fulquet E, Florez S, Echevarria JR, Fiz L (2007) Plasma troponins as markers of myocardial damage during cardiac surgery with extracorporeal circulation. Tohoku J Exp Med 213:63–69
Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW (2012) A systematic review and meta-analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J R Soc Med 105:436–445
Jaffe AS, Wu AH (2012) Troponin release-reversible or irreversible injury? Should we care? Clin Chem 58:148–150
Lehrke S, Steen H, Sievers HH, Peters H, Opitz A, Muller-Bardorff M, Wiegand UK, Katus HA, Giannitsis E (2004) Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem 50:1560–1567
Payne RE, Aldwinckle J, Storrow J, Kong RS, Lewis ME (2015) RIPC remains a promising technique for protection of the myocardium during open cardiac surgery: a meta-analysis and systematic review. Heart Surg Forum 18:E074–E080
Tie HT, Luo MZ, Li ZH, Wang Q, Wu QC, Li Q, Zhang M (2015) Remote ischemic preconditioning fails to benefit pediatric patients undergoing congenital cardiac surgery: a meta-analysis of randomized controlled trials. Medicine 94:e1895
Bangalore H, Gaies M, Ocampo EC, Heinle JS, Guffey D, Minard CG, Checchia P, Shekerdemian LS (2017) The total inotrope exposure score: an extension of the vasoactive inotrope score as a predictor of adverse outcomes after paediatric cardiac surgery. Cardiol Young 27(6):1146–1152
Agarwal HS, Wolfram KB, Saville BR, Donahue BS, Bichell DP (2014) Postoperative complications and association with outcomes in pediatric cardiac surgery. J Thorac Cardiovasc Surg 148:609–616
Heusch G (2017) Remote ischemic conditioning in cardiovascular surgery. J Cardiovasc Pharmacol Ther 22:297–301
Yang L, Wang G, Du Y, Ji B, Zheng Z (2014) Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth 28:682–689
Luo SJ, Zhou YJ, Shi DM, Ge HL, Wang JL, Liu RF (2013) Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol 29:1084–1089
Wang S, Li H, He N, Sun Y, Guo S, Liao W, Liao Y, Chen Y, Bin J (2017) Impact of remote ischaemic preconditioning on major clinical outcomes in patients undergoing cardiovascular surgery: a meta-analysis with trial sequential analysis of 32 randomised controlled trials. Int J Cardiol 227:882–891
Zhou C, Bulluck H, Fang N, Li L, Hausenloy DJ (2017) Age and surgical complexity impact on renoprotection by remote ischemic preconditioning during adult cardiac surgery: a meta analysis. Sci Rep 7:215
Pei H, Wu Y, Wei Y, Yang Y, Teng S, Zhang H (2014) Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta-analysis of 11 randomized trials. PLoS ONE 9:e115500
Pierce B, Bole I, Patel V, Brown DL (2017) Clinical outcomes of remote ischemic preconditioning prior to cardiac surgery: a meta-analysis of randomized controlled trials. J Am Heart Assoc 6(2):e004666
Zhou C, Liu Y, Yao Y, Zhou S, Fang N, Wang W, Li L (2013) Beta-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J Cardiothorac Vasc Anesth 27:305–311
Stokfisz K, Ledakowicz-Polak A, Zagorski M, Zielinska M (2017) Ischaemic preconditioning—current knowledge and potential future applications after 30 years of experience. Adv Med Sci 62:307–316
Costa FL, Yamaki VN, Goncalves TB, Coelho JV, Percario S, Brito MV (2014) Combined remote ischemic preconditioning and local postconditioning on liver ischemia-reperfusion injury. J Surg Res 192:98–102
Hong DM, Jeon Y, Lee C-S, Kim HJ, Lee J-M, Bahk J-H, Kim K-B, Hwang HY (2012) Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery. Circ J 76:884–890
Zhong H, Gao Z, Chen M, Zhao J, Wang F, Li L, Dong H, Liu L, Wang Q, Xiong L (2013) Cardioprotective effect of remote ischemic postconditioning on children undergoing cardiac surgery: a randomized controlled trial. Paediatr Anaesth 23:726–733
Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ (2007) Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 116:1386–1395
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Wen Tan, Chaoji Zhang, Jianzhou Liu, Xiaofeng Li, Yuzhi Chen, Qi Miao declare that they have no conflicts of interest in the meta-analysis.
Research Involving with Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors
Rights and permissions
About this article
Cite this article
Tan, W., Zhang, C., Liu, J. et al. Remote Ischemic Preconditioning has a Cardioprotective Effect in Children in the Early Postoperative Phase: A Meta-Analysis of Randomized Controlled Trials. Pediatr Cardiol 39, 617–626 (2018). https://doi.org/10.1007/s00246-017-1802-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1802-7