Abstract
Purpose
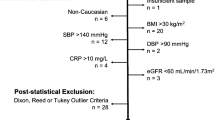
Laboratory reference intervals (RIs) play a key role in clinical pharmacology trials, both in the screening process and in evaluating drug safety. However, RIs tend to be confined to the general population, and data about RIs for the trial population are limited. The purpose of this study was to determine appropriate RIs for use when screening a defined special subgroup of a healthy Chinese population in clinical pharmacology trials.
Methods
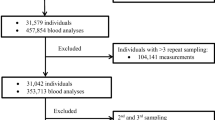
A total of 773 healthy Chinese volunteers (552 men and 221 women) who sought to participate in clinical pharmacology trials were included in this study. Sixteen different biochemical analytes were measured by a Beckman Coulter Unicel DxC 800 automatic analyzer. RIs were partitioned by gender using Harris and Boyd’s method and calculated using a non-parametric method.
Results
The RIs of 16 biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials are reported in this study. Noticeable differences between the RIs in this study and RIs provided by our laboratory or existing literature were also observed. Compared to our institutional RIs, the newly established RIs were more applicable to the current trial population.
Conclusions
The RIs in this study can serve as a powerful clinical tool during the screening process in clinical pharmacology trials. However, these RIs should be re-verified if any condition changes. The results also emphasize the importance re-establishing RIs which are more applicable to local trial populations.
Similar content being viewed by others
References
Sibille M, Deigat N, Durieu I et al (1999) Laboratory data in healthy volunteers: reference values, reference changes screening and laboratory adverse event limits in phase I clinical trials. Eur J Clin Pharmacol 55:13–19. https://doi.org/10.1007/s002280050586
Breithaupt-Groegler K, Coch C, Coenen M et al (2017) Who is a ‘healthy subject’?-consensus results on pivotal eligibility criteria for clinical trials. Eur J Clin Pharmacol 73:409–416. https://doi.org/10.1177/1740774517722130
Miller WG, Horowitz GL, Ceriotti F et al (2016) Reference intervals: strengths, weaknesses, and challenges. Clin Chem 62:916–923. https://doi.org/10.1373/clinchem.2016.256511
Ozarda Y (2016) Reference intervals: current status, recent developments and future considerations. Biochem Med 26:5–16. https://doi.org/10.11613/BM.2016.001
Friedberg RC, Souers R, Wagar EA et al (2007) The origin of reference intervals. Arch Pathol Lab Med 131:348–357. https://doi.org/10.1043/1543-2165(2007)131[348:TOORI]2.0.CO;2
Shine B (2008) Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem 45:467–475. https://doi.org/10.1258/acb.2008.008028
Mekonnen Z, Amuamuta A, Mulu W et al (2017) Clinical chemistry reference intervals of healthy adult populations in Gojjam zones of Amhara National Regional State, Northwest Ethiopia. PLoS One 12:e0184665. https://doi.org/10.1371/journal.pone.0184665
Rustad P, Felding P, Lahti A et al (2004) Descriptive analytical data and consequences for calculation of common reference intervals in the Nordic reference interval project 2000. Scand J Clin Lab Invest 64:343–370. https://doi.org/10.1080/00365510410006306
Mu R, Chen W, Pan B et al (2013) First definition of reference intervals of liver function tests in China: a large-population-based multi Center study about healthy adults. PLoS One 8:e72916. https://doi.org/10.1371/journal.pone.0072916
Lee SG, Lee W, Kim JH et al (2012) Gender-specific reference intervals for serum total bilirubin in healthy Korean adults. Clin Biochem 45:1257–1259. https://doi.org/10.1016/j.clinbiochem.2012.06.033
International Organization for Standardization. ISO 15189:2012 Medical laboratories- Requirements for quality and competence, 3rd ed., 2012
Ozarda Y, Ichihara K, Aslan D et al (2014) A multicenter nationwide reference intervals study for common biochemical analytes in Turkey using Abbott analyzers. Clin Chem Lab Med 52:1823–1833. https://doi.org/10.1515/cclm-2014-0228
Jones GRD, Haeckel R, Loh TP et al (2018) Indirect methods for reference interval determination-review and recommendations. Clin Chem Lab Med 57:20–29. https://doi.org/10.1515/cclm-2018-0073
Grady C, Bedarida G, Sinaii N et al (2017) Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research. Clin Trials 14:526–536. https://doi.org/10.1177/1740774517722130
den Elzen WP, van Gerven J, Schenk PW et al (2017) How to define reference intervals to rule in healthy individuals for clinical trials? Clin Chem Lab Med 55:e59–e61. https://doi.org/10.1515/cclm-2016-0307
Clinical and Laboratory Standards Institute (CLSI) (2010) Defifining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, CLSI document C28-A3, Third edition
Ichihara K, Ceriotti F, Tam TH, S et al (2013) The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med 51: 1429-1442. https://doi.org/10.1515/cclm-2012-0421
Shah SAV, Ichihara K, Dherai AJ et al (2018) Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative. Clin Chem Lab Med 56:2093–2103. https://doi.org/10.1515/cclm-2018-0152
Borai A, Ichihara K, Al Masaud A et al (2016) Establishment of reference intervals of clinical chemistry analytes for adult population in Saudi Arabia: a study conducted as a part of the IFCC global study on reference values. Clin Chem Lab Med 54:843–855. https://doi.org/10.1515/cclm-2015-0490
Xia L, Chen M, Liu M et al (2016) Nationwide Multicenter reference interval study for 28 common biochemical Analytes in China. Medicine 95:e2915. https://doi.org/10.1097/MD.0000000000002915
Yang S, Qiao R, Li Z et al (2012) Establishment of reference intervals of 24 chemistries in apparently healthy adult Han population of northern China. Clin Biochem 45:1213–1218. https://doi.org/10.1016/j.clinbiochem.2012.06.022
Iltis AS (2009) Payments to normal healthy volunteers in phase 1 trials: avoiding undue influence while distributing fairly the burdens of research participation. J Med Philos 34:68–90. https://doi.org/10.1093/jmp/jhn036
Almeida L, Azevedo B, Nunes T et al (2007) Why healthy subjects volunteer for phase I studies and how they perceive their participation. Eur J Clin Pharmacol 63:1085–1094. https://doi.org/10.1007/s00228-007-0368-3
Stones M, McMillan J (2010) Payment for participation in research: a pursuit for the poor? J Med Ethics 36:34–36. https://doi.org/10.1136/jme.2009.030965
Abebe M, Melku M, Enawgaw B et al (2018) Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia. PLoS One 13:e0201782. https://doi.org/10.1371/journal.pone.0201782
Zeh C, Amornkul PN, Inzaule S et al (2011) Population-based biochemistry, immunologic and hematological reference values for adolescents and young adults in a rural population in Western Kenya. PLoS One 6:e21040. https://doi.org/10.1371/journal.pone.0021040
Tembe N, Joaquim O, Alfai E et al (2014) Reference values for clinical laboratory parameters in young adults in Maputo, Mozambique. PLoS One 9:e97391. https://doi.org/10.1371/journal.pone.0097391
Adeli K, Higgins V, Nieuwesteeg M et al (2015) Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian health measures survey. Clin Chem 61:1049–1062. https://doi.org/10.1373/clinchem.2015.240515
Claridge LC, Armstrong MJ, Booth C et al (2011) Gilbert's syndrome. BMJ 342:d2293. https://doi.org/10.1136/bmj.d2293
Horsfall LJ, Nazareth I, Pereira SP et al (2013) Gilbert's syndrome and the risk of death: a population-based cohort study. J Gastroenterol Hepatol 28:1643–1647. https://doi.org/10.1111/jgh.12279
Cirillo M, Lombardi C, Chiricone D et al (2014) Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant 29:1733–1740. https://doi.org/10.1093/ndt/gfu056
Rowland R, O'Hara GA, Hamill M et al (2013) Determining the validity of hospital laboratory reference intervals for healthy young adults participating in early clinical trials of candidate vaccines. Hum Vaccin Immunother 9:1741–1751. https://doi.org/10.4161/hv.24998
Murakami K, Sasaki S, Takahashi Y et al (2009) Neighborhood socioeconomic disadvantage is associated with higher ratio of 24-hour urinary sodium to potassium in young Japanese women. J Am Diet Assoc 109:1606–1611. https://doi.org/10.1016/j.jada.2009.06.391
Eller LA, Eller MA, Ouma B et al (2008) Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS One 3:e3919. https://doi.org/10.1371/journal.pone.0003919
Ichihara K (2014) Statistical considerations for harmonization of the global multicenter study on reference values. Clin Chim Acta 432:108–118. https://doi.org/10.1016/j.cca.2014.01.025
Acknowledgments
The authors are very grateful to Dr. Wenling Zhu for her dedicated contribution to the assessment of the health status of participants in this study. In addition, the authors are very grateful to Dr. Songxue Guo for his dedicated contribution to the English language editing on the manuscript.
Funding
This work was supported by the following grants: National Natural Science Foundation of China (NSFC) Grants 81671909, 81901958 and Zhejiang Provincial Natural Science Foundation of China Grants LY18H150004, LY19H150004, LY20H150010.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: PZ, XL. Performed the experiments: PZ, HC, XL JL. Analyzed the data: PZ, HC. Wrote the paper: PZ, XL. Initiated and organized this project: PZ. All authors reviewed and edited the manuscript and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, P., Chen, H., Luo, X. et al. Establishment of reference intervals of biochemical analytes for healthy Chinese volunteers during the screening process in clinical pharmacology trials. Eur J Clin Pharmacol 76, 1227–1235 (2020). https://doi.org/10.1007/s00228-020-02912-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02912-1