Abstract
Objective
The aim of this study was to investigate the safety, maximum tolerated dose and pharmacokinetics (PK) of iguratimod and the effect of food on PK parameters in healthy adult volunteers.
Methods
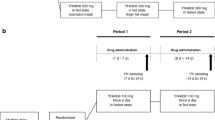
This phase 1 study consisted of four parts. Part 1 was a single-ascending dose (3.125, 6.25, 12.5, 25, 50, 75 mg) study to assess the maximum tolerated dose and safety of iguratimod. Part 2 was a single-ascending dose study to analyze the pharmacokinetic (PK) parameters of iguratimod; subjects were divided into three groups, with each group receiving iguratimod at a different dose (25, 50 or 75 mg). Part 3 was designed to compare the pharmacokinetic parameters of iguratimod between single-dose and multiple-dose administration; subjects were divided into two groups, with one group receiving a single dose of 50 mg on day 1 and the other group receiving a multiple dose of 50 mg, once every day, until a stable plasma concentration had been achieved. The aim of part 4 was to evaluate the effect of food on the pharmacokinetic parameters of iguratimod; subjects were divided into two groups, namely a fed group and a fasted group, with each group receiving a single 50 mg dose of iguratimod on day 1. Following a 14-day washout period, the two groups were crossed-over and received a single dose of 50 mg iguratimod on day 15.
Results
In part 1 of the study, iguratimod at doses ranging from 3.125 to 50 mg were well tolerated, with most adverse effects (AEs) being mild; no severe AEs occurred. In part 2, there were no significant differences in Tmax, T1/2, Ka and V/F among volunteers receiving doses of 25, 50 and 75 mg iguratimod. The Cmax and AUC0-last in volunteers receiving 75 mg iguratimod were higher than those in volunteers receiving 25 and 50 mg. The Cmax was linear from 25 to 75 mg, with a correlation coefficient (r 2) of 0.9808. The AUC0-last was also linear from 25 to 75 mg, with an r 2 of 0.9839. In part 3, in subjects receiving multiple doses of 50 mg, the T1/2 was 10.25 h, Tmax was 3.63 h, Cmax was 1.88 mg/L, AUC0-last was 31.88 mg/L h, Vd was 1.16 L and Ka was 0.87 1/h.There were no significant differences in the Cmax, AUC0-last, Ka and V/F between the single-dose and multiple-dose groups; there were, however, significant differences in Tmax and T1/2 between the two groups. In part 4, there were no significant differences in T1/2, AUC0-last, Ka and V/F between the fed group and fasted group; however, food may promote the absorption of iguratimod.
Conclusions
The maximum tolerated dose for iguratimod was confirmed to be 50 mg. The ingestion of food was able to increase the peak concentration of iguratimod and shorten the time to peak concentration. Therefore, based on our results, iguratimod can be administered with food. The PK profile and metabolic effects of iguratimod support further clinical development for its application in treating autoimmune diseases.




Similar content being viewed by others
References
Tanaka K, Shimotori T, Makino S, Aikawa Y, Inaba T, Yoshida C, Takano S (1992) Pharmacological studies of the new anti-inflammatory agent 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one. 1st communication: antiinflammatory, analgesic and other related properties. Arzneimittelforschung 42:935–944
Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F, Gu Y, Wu X, Shen Y, Xu Q (2013) A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol 191:4969–4978
Ishiguro N, Yamamoto K, Katayama K, Kondo M, Sumida T, Mimori T, Soen S, Nagai K, Yamaguchi T, Hara M, Iguratimod-Clinical Study Group (2013) Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod Rheumatol 23:430–439
Zhou T, Ding L, Li X, Zhang F, Zhang Q, Gong B, Guo X (2008) Determination of iguratimod in rat plasma by high performance liquid chromatography: method and application. Biomed Chromatogr 22:260–264
Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M, Nakano S (2007) Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol 17:1–9
Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M (2007) Long-term safety study of iguratimod in patients with rheumatoid arthritis. Mod Rheumatol 17:10–16
Du F, Lü LJ, Teng JL, Shen N, Ye P, Bao CD (2012) T-614 alters the production of matrix metalloproteinases (MMP-1 and MMP-3) and inhibits the migratory expansion of rheumatoid synovial fibroblasts, in vitro. Int Immunopharmacol 13:54–60
Tanaka K, Urata N, Mikami M, Ogasawara M, Matsunaga T, Terashima N, Suzuki H (2007) Effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. Inflamm Res 56:17–23
Yoshioka Y, Takahashi N, Kaneko A, Hirano Y, Kanayama Y, Kanda H, Takagi H, Ito T, Kato T, Saito K, Funahashi K, Asai S, Takemoto T, Terabe K, Asai N, Ishiguro N, Kojima T (2016) Disease activity early in treatment as a predictor of future low disease activity in RA patients treated with iguratimod. Mod Rheumatol 26:169–174
Okamura K, Yonemoto Y, Suto T, Okura C, Takagishi K (2015) Efficacy at 52 weeks of daily clinical use of iguratimod in patients with rheumatoid arthritis. Mod Rheumatol 25:534–539
Okamura K, Yonemoto Y, Okura C, Kobayashi T, Takagishi K (2015) Efficacy of the clinical use of iguratimod therapy in patients with rheumatoid arthritis. Mod Rheumatol 25:235–240
Zhang J, Yuan Y, Li JH, Wang YY, Liu GY (2007) Pharmacokinetics study of iguratimod in Chinese healthy adult volunteers. Chin J Clin Pharmacy 26(4):78–80
Lin CC, Philips L, Xu C, Yeh LT (2004) Pharmacokinetics and safety of viramidine, a prodrug of ribavirin, in healthy volunteers. J Clin Pharmacol 44(3):265–275
Kawakami A, Tsuboi M, Urayama S, Matsuoka N, Yamasaki S, Hida A et al (1999) Inhibitory effect of a new anti-rheumatic drug T-614 on costimulatory molecule expression, cytokine production, and antigen presentation by synovial cells. J Lab Clin Med 133:566–574
Kohno M, Aikawa Y, Tsubouchi Y, Hashiramoto A, Yamada R, Kawahito Y et al (2001) Inhibitory effect of T-614 on tumor necrosis factor-alpha induced cytokine production and nuclear factor kappa B activation in cultured human synovial cells. J Rheumatol 28:2591–2596
Aikawa Y, Yamamoto M, Yamamoto T, Morimoto K, Tanaka K (2002) An anti-rheumatic agent T-614 inhibits NF-kappaB activation in LPS- and TNF-alpha-stimulated THP-1 cells without interfering with IkappaBalpha degradation. Inflamm Res 51:188–194
Tanaka K, Yamamoto T, Aikawa Y, Kizawa K, Muramoto K, Matsuno H et al (2003) Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology 42:1365–1371
Lu LJ, Bao CD, Dai M, Teng JL, Fan W, Du F et al (2009) Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum 61:979–987
Acknowledgements
This work was financially supported through the National Natural Science Foundation of China (No 81330081, 81473223), China Postdoctoral Science Foundation (NO 2013 M540509), the Anhui Province Natural Science Foundation in University (No. KJ2013Z145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All subjects gave written informed consent to participate in this study. All experiments were approved by an independent Ethics Committee and carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Rights and permissions
About this article
Cite this article
Xiao, F., Zhang, F., Zhang, Ll. et al. A randomized phase I study to evaluate the safety, tolerability, pharmacokinetics and food-effect of Iguratimod in healthy adult volunteers. Eur J Clin Pharmacol 74, 69–77 (2018). https://doi.org/10.1007/s00228-017-2342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2342-z