Abstract
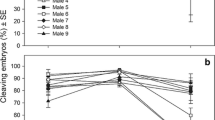
Breeding designs such as the North Carolina II can be used to identify the sources of genetic and environmental variances in embryo performance. Here this approach is used for the Antarctic sea urchin S. neumayeri to explore how the contribution of sire and dam can influence the performance of cleavage stage embryos and blastulae, and how these contributions differ when exposed to stress from increased temperature and acidification. The interrelationship of sire–dam effects was also compared across developmental stages. The effects of warming (+3 °C) and acidification (−0.3 and −0.5 pHT units) on 24 sire–dam crosses were investigated. These stressors decreased cleavage success and the percentage of normal blastulae, with a negative interactive effect between stressors. The response to these factors differed among the sire–dam pairs indicating the influence of gamete compatibility. A positive genetic correlation indicated that genotypes that performed well as blastulae in low pH also performed well at increased temperatures. Performance at cleavage was a good predictor of performance at the later blastula stage. Significant dam by temperature interactions indicated differential performance among maternal half-siblings in response to increased temperature. Adaptation depends on additive genetic variance for stress tolerance being present in populations; however, there were no sire by stressor interactions found. This indicates that S. neumayeri will need to rely on phenotypic plasticity to persist through an ocean decreasing in pH and warming, at least with respect to early development.





Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. Primer-E Ltd, Plymouth
Astles PA, Moore AJ, Preziosi RF (2006) Comparison of methods to estimate cross-environment genetic correlations. J Evol Biol 19:114–122
Bell G (2013) Evolutionary rescue and the limits of adaptation. Philos Trans Roy Soc B 368:20120080
Billington HL, Pelham J (1991) Genetic variation in the date of budburst in Scottish birch populations: implications for climate change. Funct Ecol 5:403–409
Bosch I, Beauchamp KA, Steele ME, Pearse JS (1987) Development, metamorphosis, and seasonal abundance of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri. Biol Bull 173:126–135
Brockington S, Peck LS, Tyler PA (2007) Gametogenesis and gonad mass cycles in the common circumpolar Antarctic echinoid Sterechinus neumayeri. Mar Ecol Prog Ser 330:139–147
Byrne M, Ho M, Koleits L, Price C, King C, Virtue P, Tilbrook B, Lamare M (2013) Vulnerability of the calcifying larval stage of the Antarctic sea urchin Sterechinus neumayeri to near-future ocean acidification and warming. Glob Change Biol 19:2264–2275
Clark D, Lamare M, Barker M (2009) Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar Biol 156:1125–1137
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer Assoc. Inc., Sunderland
Crean AJ, Bonduriansky R (2014) What is a paternal effect? Trends Ecol Evol 29:554–559
Dickson AG, Millero FJA (1987) Comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34:1733–1743
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean O2 measurements. PICES special publication, 3, 119 pp
Durrant HMS, Clark GF, Dworjanyn SA, Byrne M, Johnston E (2013) Seasonal variation in the effects of ocean warming and acidification on a native bryozoan, Celleporaria nodulosa. Mar Biol 160:1903–1911
Eisen EJ, Saxton AM (1983) Genotype by environment interactions and genetic correlations involving two environmental factors. Theor Appl Genet 67:75–86
Enzor LA, Zippay ML, Place SP (2013) High latitude fish in a high CO2 world: synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comp Biochem Phys A 164:154–161
Ericson JA, Lamare MD, Morley SA, Barker MF (2010) The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH, effects on fertilisation and embryonic development. Mar Biol 157:2689–2702
Ericson JA, Ho MA, Miskelly A, King CK, Virtue P, Tilbrook B, Byrne M (2012) Combined effects of two ocean change stressors, warming and acidification, on fertilisation and early development of the Antarctic echinoid Sterechinus neumayeri. Polar Biol 35:1027–1034
Etterson JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 294:151–154
Evans JP, Garcia-Gonzalez F, Marshall DJ (2008) Sources of genetic and phenotypic variance in fertilization rates and larval traits in a sea urchin. Evolution 61:2832–2838
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Falconer DS (1989) Introduction to quantitative genetics. Longman, New York
Foltz KR, Adams NL, Runft LL (2004) Echinoderm eggs and embryos: procurement and culture. Methods Cell Biol 74:39–74
Foo SA, Dworjanyn SA, Poore AGB, Byrne M (2012) Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: performance of early embryos. PLoS ONE 7:e42497
Foo SA, Dworjanyn SA, Poore AGB, Byrne M (2014) Increased temperature, but not acidification, enhances fertilisation and development in a tropical urchin: potential for adaptation to a tropicalized eastern Australia. Evol Appl 7:1226–1237
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Hamdoun A, Epel D (2007) Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA 104:1745–1750
Harrison PM, Gerstein M (2002) Studying genomes through the aeons: protein families, pseudogenes and proteome evolution. J Mol Biol 318:1155–1174
Hart MW, Strathmann RR (1994) Functional consequences of phenotypic plasticity in echinoid larvae. Biol Bull 186:291–299
Ho MA, Price C, King CK, Virtue P, Byrne M (2013) Effects of ocean warming and acidification on fertilisation in the Antarctic echinoid Sterechinus neumayeri across a range of sperm concentrations. Mar Environ Res 90:136–141
Hoffmann AA, Sgro CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Hofmann GE, Evans TG, Kelly MW, Padilla-Gamino JL, Blanchette CA, Washburn L, Chan F, McManus MA, Menge BA, Gaylord B et al (2013) Exploring local adaptation and the ocean acidification seascape—studies in the California current large marine ecosystem. Biogeosci Discuss 10:11825–11856
IPCC (2014) Climate change 2014: synthesis report. In: Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
Jensen N, Allen RM, Marshall DJ (2014) Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Funct Ecol 28:724–733
Kapsenberg L, Hofmann GE (2014) Signals of resilience to ocean change: high thermal tolerance of early stage Antarctic sea urchins (Sterechinus neumayeri) reared under present-day and future pCO2 and temperature. Polar Biol 37:967–980
Kapsenberg L, Kelley AL, Shaw EC, Martz TR, Hofmann GE (2015) Near-shore Antarctic pH variability has implications for the design of ocean acidification experiments. Sci Rep 5. doi:10.1038/srep09638
Kelly MW, Sanford E, Grosberg RK (2011) Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc R Soc B 279:349–356
Kelly MW, Padilla-Gamino JL, Hofmann GE (2013) Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob Change Biol 19:2536–2546
Lamare MD, Barker MF (1999) In situ estimates of larval development and mortality in the New Zealand sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). Mar Environ Prog Ser 180:197–211
Lister KN, Lamare MD, Burritt DJ (2015) Pollutant resilience in embryos of the Antarctic sea urchin Sterechinus neumayeri reflects maternal antioxidant status. Aquat Toxicol 161:61–72
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Sunderland
Marshall DJ (2015) Environmentally induced (co)variance in sperm and offspring phenotypes as a source of epigenetic effects. J Exp Biol 208:107–113
Marshall DJ, Keough MJ (2008) The evolutionary ecology of offspring size in marine invertebrates. Adv Mar Biol 53:1–60
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Microsoft (2013) Microsoft Excel: Computer software. Microsoft, Redmond
Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ (2013) Predicting evolutionary responses to climate change in the sea. Ecol Lett 16:1488–1500
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Palumbi SR (1999) All sires are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc Natl Acad Sci USA 96:12632–12637
Peck LS (2005) Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarct Sci 17:497–507
Peck LS (2015) A cold limit to adaptions. Trends Ecol Evol 31:13–26
Peck LS, Souster T, Clark M (2013) Juveniles are more resistant to warming than adults in 4 species of Antarctic marine invertebrates. PLoS ONE 8:e66033
Pierrot D, Lewis E, Wallace DWR (2006) MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge
Pörtner HO, Peck LS, Somero GN (2007) Thermal limits and adaptation: an integrative view (Antarctic ecology: from genes to ecosystems). Philos Trans R Soc B 362:2233–2258
Quinn G, Keough M (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Räsänen K, Kruuk LEB (2007) Maternal effects and evolution at ecological time scales. Funct Ecol 21:408–421
Schlegel P, Havenhand JN (2012) Individual variability in reproductive success determines winners and losers under ocean acidification: a case study with sea urchins. PLoS ONE 7:e53118
Sewell MA, Millar RB, Yu PC, Kapsenberg L, Hofmann GE (2014) Ocean acidification and fertilisation in the antarctic sea urchin Sterechinus neumayeri: the importance of polyspermy. Environ Sci Technol 48:713–722
Sgro CM, Blows MW (2004) The genetic covariance among clinal environments after adaptation to an environmental gradient in Drosophila serrata. Genetics 167:1281–1291
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Suckling CC, Clark MS, Richard J, Morley SA, Thorne MAS, Harper EM, Peck LS (2015) Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J Anim Ecol 84:773–784
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6:e22881
Sunday JM, Calosi P, Dupont S, Munday PL, Stillman JH, Reusch TBH (2014) Evolution in an acidifying ocean. Trends Ecol Evol 29:117–125
Turley C, Blackford J, Hardman-Mountford N, Litt E, Llewellyn C, Lowe D, Miller P, Nightingale P, Rees A, Smyth T, Tilstone G, Widdicombe S (2010) Carbon uptake, transport and storage by oceans and the consequences of change in carbon capture and storage. In: Harrison R, Hester R (eds) Issues in environmental science and technology. Royal Society of Chemistry, UK, pp 240–284
Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39:505–522
Yu PC, Sewell MA, Matson PG, Rivest EB, Kapsenberg L et al (2013) Growth attenuation with developmental schedule progression in embryos and early larvae of Sterechinus neumayeri raised under elevated CO2. PLoS ONE 8:e52448
Acknowledgments
Research was supported by a New Zealand Antarctic Research Institute Grant (MDL) and a University of Sydney Ph.D. Scholarship (SF). We thank Antarctica New Zealand for logistical support, and are grateful for sea urchin collections made by Mr. Rob Robbins (USAP) and Professor Steve Wing (University of Otago). Dr. Kim Currie (University of Otago) supervised the analysis of water samples. We thank two reviewers for valuable feedback.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Stillman.
Reviewed by M. Kelly and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foo, S.A., Sparks, K.M., Uthicke, S. et al. Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Mar Biol 163, 130 (2016). https://doi.org/10.1007/s00227-016-2903-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2903-1